What is AgCl Compound Name?

Opening Paragraph
AgCl, or silver chloride, is a chemical compound that has garnered significant attention in various fields, including chemistry, photography, and medicine. Its unique properties make it a versatile substance with both informational and commercial value. Whether you’re a student, researcher, or industry professional, understanding AgCl is essential. This post will delve into what AgCl is, its properties, applications, and how it can be utilized effectively. (AgCl compound name, silver chloride uses, chemical properties of AgCl)
What is AgCl Compound Name?

AgCl stands for silver chloride, a white crystalline solid composed of silver (Ag) and chlorine (Cl) ions. It is one of the most well-known silver compounds due to its insolubility in water and sensitivity to light. These characteristics make it a crucial component in various industries. (AgCl compound name, silver chloride formula, chemical composition)
Chemical Formula and Structure
The chemical formula of silver chloride is AgCl, representing one silver ion (Ag⁺) and one chloride ion (Cl⁻) bonded together. Its crystal structure is face-centered cubic, which contributes to its stability and unique properties. (silver chloride formula, crystal structure of AgCl, ionic bonding)
📌 Note: AgCl is highly insoluble in water, making it a key compound in qualitative analysis and precipitation reactions.
Properties of AgCl

Understanding the properties of AgCl is crucial for its applications. Below are its key characteristics:
Physical Properties
- Color: White crystalline solid
- Solubility: Insoluble in water, slightly soluble in concentrated ammonia
- Melting Point: 455°C (851°F)
- Density: 5.56 g/cm³
Chemical Properties
- Photosensitivity: Decomposes into silver and chlorine gas when exposed to light
- Reactivity: Reacts with acids to form soluble silver salts
- Stability: Stable under normal conditions but decomposes at high temperatures
| Property | Value |
|---|---|
| Chemical Formula | AgCl |
| Molecular Weight | 143.32 g/mol |
| Solubility in Water | 0.0005 g/100 mL |
Applications of AgCl
AgCl’s unique properties make it valuable in multiple industries. Here are its primary applications:
Photography
AgCl is widely used in photographic films and papers due to its light-sensitive nature. When exposed to light, it forms metallic silver, creating images. (silver chloride in photography, light-sensitive compounds)
Medicine
In medicine, AgCl is used in antimicrobial dressings and wound care products. Its antibacterial properties help prevent infections. (silver chloride medical uses, antimicrobial agents)
Chemical Analysis
AgCl is employed in qualitative analysis to detect chloride ions in solutions. Its insolubility makes it ideal for precipitation reactions. (chemical analysis with AgCl, chloride ion detection)
How to Prepare AgCl

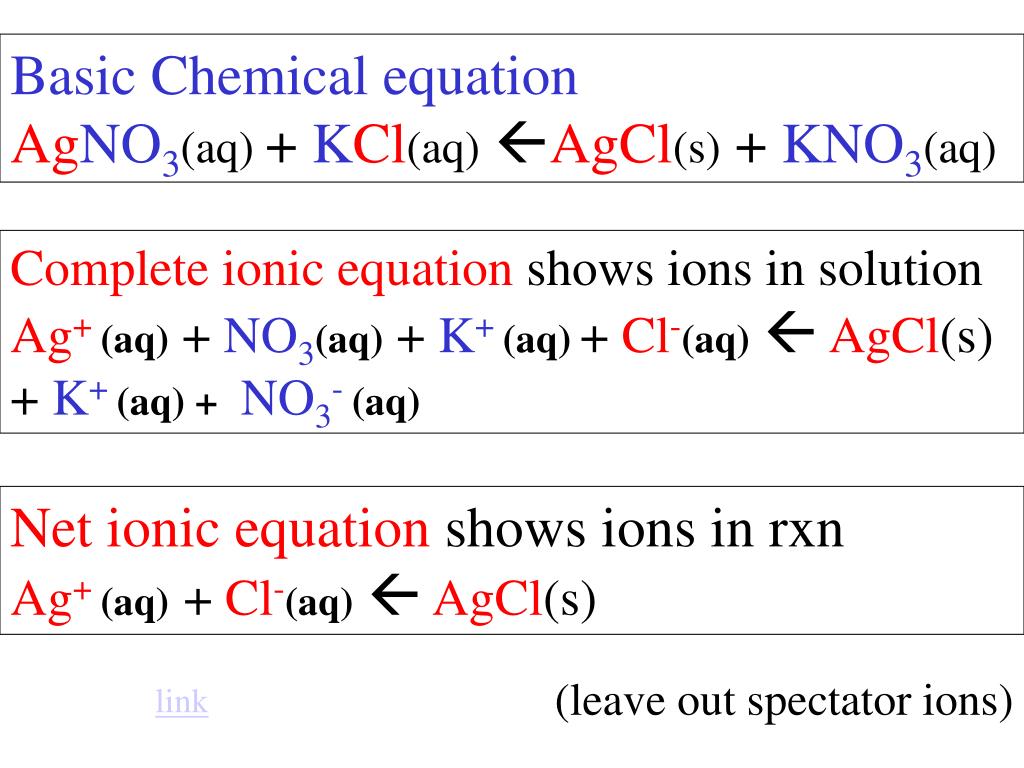
Preparing AgCl is straightforward and involves reacting silver nitrate (AgNO₃) with sodium chloride (NaCl) in water. The reaction is as follows:
AgNO₃ + NaCl → AgCl↓ + NaNO₃
The insoluble AgCl precipitates out of the solution, which can then be filtered and dried. (preparation of AgCl, silver chloride synthesis)
📌 Note: Ensure proper ventilation when handling chemicals, and dispose of waste according to safety guidelines.
Safety and Handling

While AgCl is relatively safe, it’s essential to handle it with care:
- Avoid Inhalation: Do not inhale AgCl dust.
- Skin Contact: Wear gloves to prevent skin irritation.
- Eye Protection: Use safety goggles to avoid eye contact.
Final Thoughts
AgCl, or silver chloride, is a fascinating compound with diverse applications ranging from photography to medicine. Its unique properties, such as insolubility and photosensitivity, make it indispensable in various industries. By understanding its chemical composition, properties, and uses, you can appreciate its significance in both scientific and commercial contexts. Whether you’re conducting research or exploring industrial applications, AgCl remains a compound worth knowing. (AgCl compound name, silver chloride uses, chemical properties of AgCl)
What is the chemical formula of AgCl?
+
The chemical formula of AgCl is AgCl, representing silver chloride.
Is AgCl soluble in water?
+
No, AgCl is highly insoluble in water, with a solubility of only 0.0005 g/100 mL.
What are the main uses of AgCl?
+
AgCl is used in photography, medicine, and chemical analysis due to its light sensitivity and antimicrobial properties.