Understanding Bond Angles in Trigonal Pyramidal Molecules
Understanding bond angles in trigonal pyramidal molecules is essential for anyone studying chemistry or molecular geometry. These molecules, characterized by a central atom bonded to three other atoms and a lone pair, exhibit unique properties due to their shape. The presence of a lone pair on the central atom causes repulsion, leading to a decrease in bond angles compared to trigonal planar structures. This blog will delve into the intricacies of bond angles in trigonal pyramidal molecules, providing both informative and commercial insights for readers.
What is a Trigonal Pyramidal Molecule?

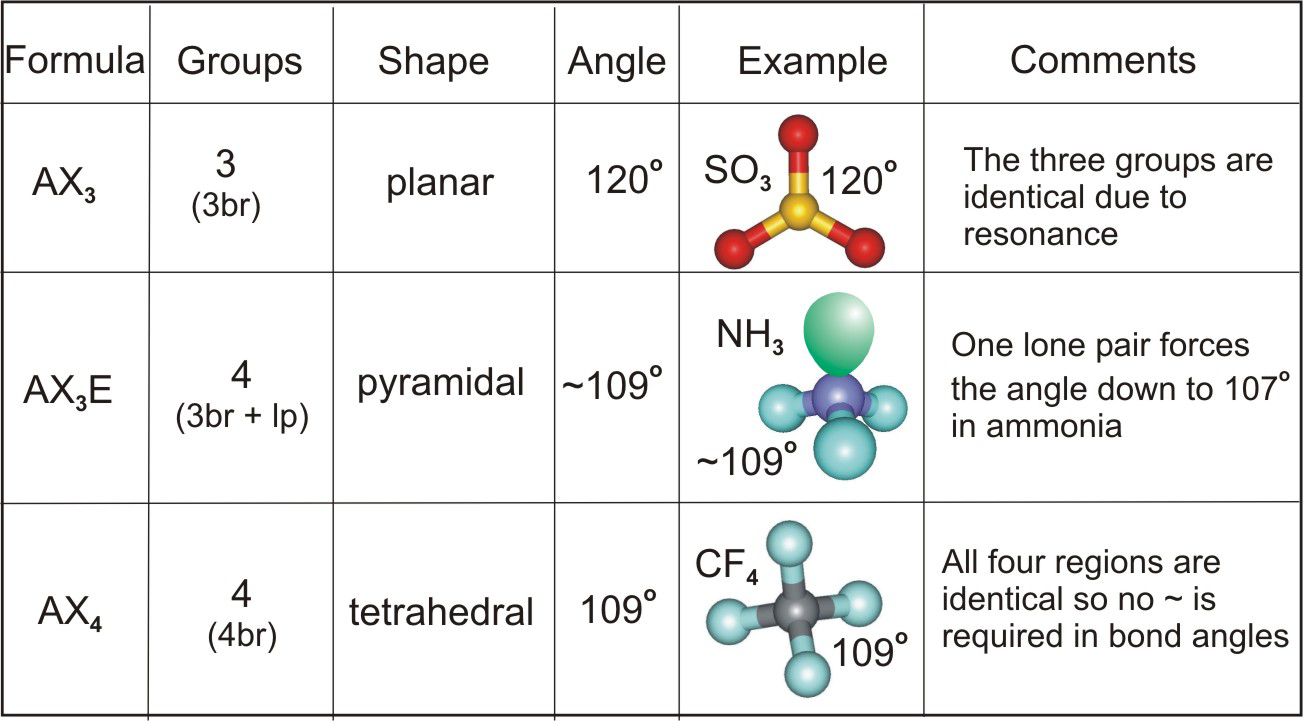
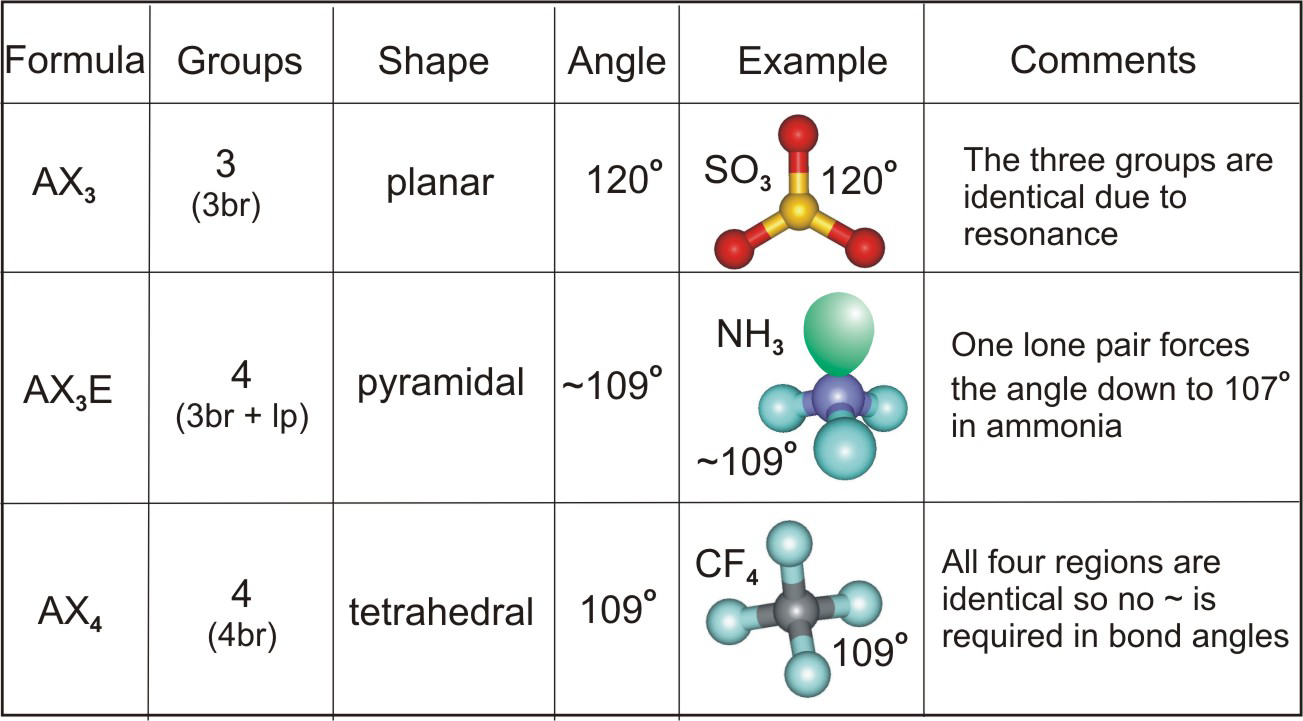
A trigonal pyramidal molecule is a type of molecular geometry where one atom is at the center, bonded to three other atoms, and has one lone pair of electrons. The lone pair repels the bonding pairs, causing the molecule to adopt a pyramid-like shape. Common examples include ammonia (NH₃) and phosphine (PH₃).
Key Characteristics of Trigonal Pyramidal Molecules
- Central Atom: Typically has a lone pair.
- Bond Angles: Approximately 107°, less than the 120° in trigonal planar molecules.
- Examples: NH₃, PH₃, and other compounds with similar structures.
📌 Note: The lone pair in trigonal pyramidal molecules significantly influences bond angles and molecular polarity.
Why Are Bond Angles Important in Trigonal Pyramidal Molecules?

Bond angles determine the overall shape and properties of a molecule. In trigonal pyramidal molecules, the 107° bond angle is a direct result of the lone pair’s repulsion. This angle affects:
- Molecular Polarity: Uneven electron distribution leads to polarity.
- Reactivity: Shape influences how the molecule interacts with others.
- Physical Properties: Boiling and melting points are impacted by molecular geometry.
Factors Affecting Bond Angles
- Lone Pair Repulsion: The lone pair pushes bonding pairs closer together.
- Electronegativity: Differences in electronegativity between atoms can alter angles slightly.
- Atomic Size: Larger atoms can reduce bond angles due to increased repulsion.
| Molecule | Central Atom | Bond Angle |
|---|---|---|
| Ammonia (NH₃) | Nitrogen | 107° |
| Phosphine (PH₃) | Phosphorus | ~107° |

How to Determine Bond Angles in Trigonal Pyramidal Molecules

To calculate bond angles, follow these steps:
1. Identify the Central Atom: Ensure it has a lone pair.
2. Count Bonding Pairs: Determine the number of atoms bonded to the central atom.
3. Apply VSEPR Theory: Use the Valence Shell Electron Pair Repulsion (VSEPR) theory to predict the shape.
4. Adjust for Lone Pairs: Subtract from the ideal trigonal planar angle (120°) due to lone pair repulsion.
✨ Note: VSEPR theory is a fundamental tool for predicting molecular geometry and bond angles.
Commercial Applications of Trigonal Pyramidal Molecules

Trigonal pyramidal molecules have practical applications in various industries:
- Pharmaceuticals: Ammonia derivatives are used in drug synthesis.
- Agriculture: Ammonia-based fertilizers enhance crop growth.
- Chemical Manufacturing: Phosphine is used in semiconductor production.
Investing in Molecular Geometry Tools
For commercial-intent visitors, understanding bond angles can aid in:
- Product Development: Designing molecules with specific properties.
- Quality Control: Ensuring consistent molecular structures in manufacturing.
- Research: Investing in advanced tools for molecular analysis.
Checklist for Understanding Trigonal Pyramidal Bond Angles
- Identify the central atom and its lone pair.
- Use VSEPR theory to predict the shape.
- Calculate bond angles by adjusting for lone pair repulsion.
- Analyze how bond angles affect molecular properties.
- Explore commercial applications in relevant industries.
What causes the bond angle in trigonal pyramidal molecules to be less than 120°?
+The lone pair on the central atom repels the bonding pairs, reducing the bond angle from 120° to approximately 107°.
Can trigonal pyramidal molecules be nonpolar?
+No, due to the asymmetric shape caused by the lone pair, trigonal pyramidal molecules are generally polar.
How does atomic size affect bond angles in trigonal pyramidal molecules?
+Larger atoms increase repulsion, which can slightly decrease bond angles compared to smaller atoms.
In summary, trigonal pyramidal molecules exhibit bond angles of approximately 107° due to lone pair repulsion. Understanding these angles is crucial for predicting molecular properties and exploring commercial applications. By applying VSEPR theory and analyzing examples like ammonia, you can master this fundamental concept in chemistry. Whether for academic or industrial purposes, knowledge of bond angles opens doors to innovation and discovery in molecular science. (Molecular Geometry,VSEPR Theory,Chemical Bonding)



