Carbonate Ion Lewis Structure: A Clear, Concise Guide

Understanding the carbonate ion Lewis structure is essential for students and professionals in chemistry, environmental science, and related fields. The carbonate ion, CO₃²⁻, plays a crucial role in various chemical processes, including ocean acidification, geological formations, and industrial applications. This guide provides a clear, concise explanation of its Lewis structure, ensuring you grasp the fundamentals effortlessly.
What is the Carbonate Ion?

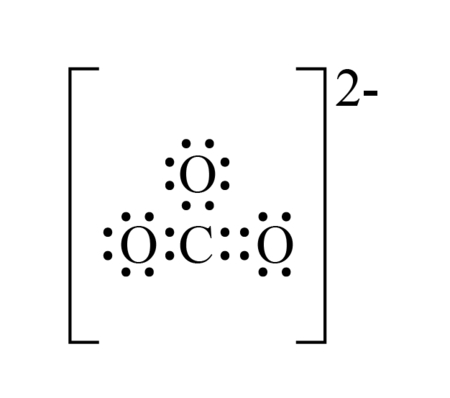
The carbonate ion (CO₃²⁻) is a polyatomic ion composed of one carbon atom and three oxygen atoms, carrying a charge of -2. It is a key component in minerals like limestone and plays a vital role in the carbon cycle.
Steps to Draw the Carbonate Ion Lewis Structure

Drawing the Lewis structure involves arranging atoms and electrons to satisfy the octet rule. Here’s a step-by-step breakdown:
Determine Total Valence Electrons
- Carbon ©: 4 electrons
- Oxygen (O): 6 electrons per atom (3 atoms = 18 electrons)
- Add 2 electrons for the -2 charge
- Total: 4 + 18 + 2 = 24 electrons
- Carbon ©: 4 electrons
Arrange Atoms
- Place the carbon atom in the center.
- Attach the three oxygen atoms around it.
- Place the carbon atom in the center.
Form Single Bonds
- Connect each oxygen atom to carbon with a single bond, using 6 electrons (3 bonds).
- Connect each oxygen atom to carbon with a single bond, using 6 electrons (3 bonds).
Complete Octets
- Distribute the remaining 18 electrons as lone pairs on the oxygen atoms.
- Distribute the remaining 18 electrons as lone pairs on the oxygen atoms.
Check Formal Charges
- Each oxygen atom has a formal charge of -1, and the carbon atom has a formal charge of +1, balancing the -2 charge of the ion.
- Each oxygen atom has a formal charge of -1, and the carbon atom has a formal charge of +1, balancing the -2 charge of the ion.
📌 Note: Resonance structures are crucial for the carbonate ion, as the double bond can be delocalized among the three oxygen atoms.
Resonance Structures of Carbonate Ion

The carbonate ion has three resonance structures, where the double bond shifts between the carbon and each oxygen atom. This delocalization of electrons stabilizes the ion.
| Resonance Structure | Double Bond Position |
|---|---|
| 1 | C=O (top oxygen) |
| 2 | C=O (left oxygen) |
| 3 | C=O (right oxygen) |
Key Takeaways
- The carbonate ion Lewis structure consists of one carbon atom and three oxygen atoms with a -2 charge.
- It has 24 valence electrons, with three resonance structures.
- Formal charges balance the overall -2 charge of the ion.
Checklist for Drawing the Carbonate Ion Lewis Structure
- [ ] Calculate total valence electrons (24).
- [ ] Place carbon in the center and attach oxygen atoms.
- [ ] Form single bonds and distribute lone pairs.
- [ ] Verify formal charges and resonance structures.
For those studying chemical bonding, Lewis structures, or polyatomic ions, mastering the carbonate ion is a foundational step. Its resonance structures and role in natural processes make it a fascinating subject in chemistry.
What is the formal charge of each atom in the carbonate ion?
+Each oxygen atom has a formal charge of -1, and the carbon atom has a formal charge of +1, totaling the -2 charge of the ion.
Why does the carbonate ion have resonance structures?
+Resonance structures allow the double bond to delocalize among the oxygen atoms, stabilizing the ion and distributing electron density evenly.
How does the carbonate ion contribute to ocean acidification?
+The carbonate ion reacts with excess CO₂ in seawater, forming bicarbonate (HCO₃⁻) and reducing ocean pH, leading to acidification.
By understanding the carbonate ion Lewis structure, you’ll gain insights into its chemical behavior and significance in various scientific disciplines. Whether for academic or professional purposes, this guide ensures clarity and precision. (Lewis structure, chemical bonding, polyatomic ions)