CH2O Boiling Point: Essential Facts Unveiled
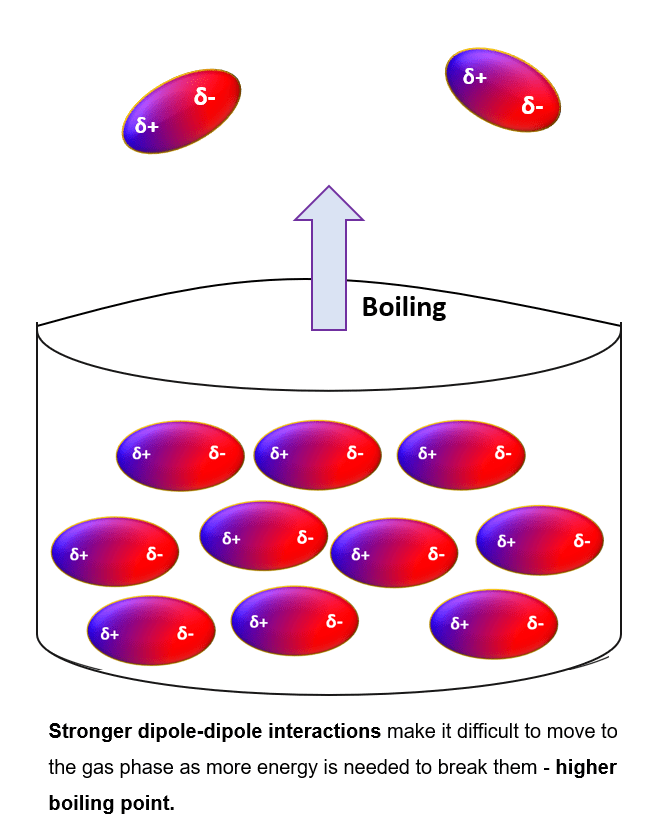
The boiling point of CH2O, commonly known as formaldehyde, is a critical piece of information for chemists, industrial professionals, and students alike. Understanding this property is essential for applications ranging from chemical synthesis to safety protocols. In this post, we’ll delve into the CH2O boiling point, its significance, and related facts that will enhance your knowledge.
What is the Boiling Point of CH2O?

Formaldehyde (CH2O) has a boiling point of -19.5°C (-3°F) under standard atmospheric pressure. This low boiling point indicates that formaldehyde is a volatile compound, readily transitioning from a liquid to a gas at temperatures slightly below room temperature.
💡 Note: The boiling point of CH2O is significantly lower than water (100°C), making it a highly volatile substance.
Why is the Boiling Point of CH2O Important?

The boiling point of CH2O is crucial for several reasons:
- Industrial Applications: It influences processes like polymer production and resin manufacturing.
- Safety Measures: Understanding its volatility helps in implementing proper handling and storage protocols.
- Chemical Reactions: Knowledge of its boiling point aids in designing experiments and reactions involving formaldehyde.
Factors Affecting CH2O Boiling Point
Several factors can alter the boiling point of formaldehyde:
- Pressure: Higher pressure increases the boiling point, while lower pressure decreases it.
- Impurities: The presence of impurities can affect the boiling point, often elevating it.
- Concentration: Solutions of CH2O in water or other solvents may exhibit different boiling points due to changes in concentration.
| Factor | Effect on Boiling Point |
|---|---|
| Pressure | Increases with higher pressure |
| Impurities | Elevates boiling point |
| Concentration | Varies based on solvent and mixture |

Practical Applications of CH2O Boiling Point

The boiling point of CH2O is vital in various industries:
- Preservation: Formaldehyde is used in preserving biological specimens due to its low boiling point and reactivity.
- Manufacturing: It plays a key role in producing materials like plastics, adhesives, and coatings.
- Laboratory Use: Researchers rely on its boiling point for purification and distillation processes.
Safety Tips When Handling CH2O

Given its volatility, handling CH2O requires caution:
- Ventilation: Work in a well-ventilated area or fume hood.
- PPE: Wear gloves, goggles, and lab coats to avoid skin and eye contact.
- Storage: Store in tightly sealed containers away from heat sources.
⚠️ Note: Prolonged exposure to formaldehyde vapor can cause respiratory issues and skin irritation.
CH2O Boiling Point vs. Other Compounds
Comparing CH2O’s boiling point with similar compounds highlights its uniqueness:
- Methanol (CH3OH): Boiling point of 64.7°C.
- Ethanol (C2H5OH): Boiling point of 78.4°C.
- Acetaldehyde (CH3CHO): Boiling point of 20.2°C.
CH2O’s lower boiling point makes it distinctively volatile compared to these compounds.
In summary, the CH2O boiling point of -19.5°C is a fundamental property with wide-ranging implications in chemistry, industry, and safety. Understanding this value and its influencing factors is essential for anyone working with formaldehyde.
What is the boiling point of CH2O?
+The boiling point of CH2O (formaldehyde) is -19.5°C (-3°F) under standard atmospheric pressure.
How does pressure affect the boiling point of CH2O?
+Higher pressure increases the boiling point of CH2O, while lower pressure decreases it.
Why is the boiling point of CH2O important in industry?
+It influences processes like polymer production, safety protocols, and chemical reactions involving formaldehyde.
Keyword/title: CH2O boiling point, formaldehyde properties, chemical safety, industrial applications, volatile compounds.


