Understanding CO32 Bond Length: Key Insights and Applications

Understanding CO3^2- Bond Length: Key Insights and Applications
The CO3^2- bond length is a fundamental concept in chemistry, crucial for understanding the structure and properties of carbonate ions. Whether you’re a student, researcher, or industry professional, grasping this topic can unlock new insights into material science, environmental chemistry, and more. This post delves into the intricacies of CO3^2- bond length, its significance, and practical applications, tailored for both informational and commercial audiences.
What is CO3^2- Bond Length?
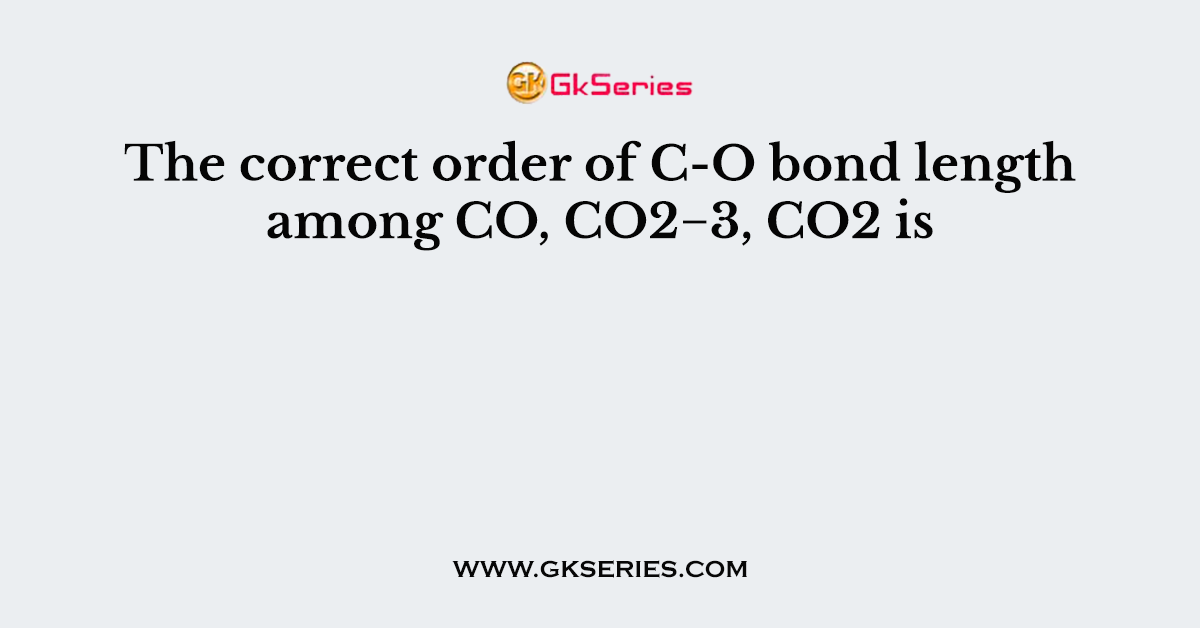
The CO3^2- bond length refers to the distance between the carbon atom and each oxygen atom in the carbonate ion (CO3^2-). This triangular anion plays a vital role in various chemical processes, from mineral formation to biological systems. Understanding its bond length is key to predicting its behavior in different environments.
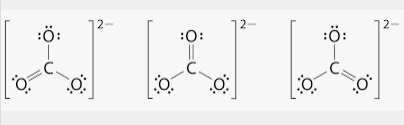
📌 Note: The CO3^2- ion has a trigonal planar geometry, resulting in equal bond lengths between carbon and oxygen atoms.
Factors Influencing CO3^2- Bond Length
Several factors affect the CO3^2- bond length, including:
- Electronegativity: Oxygen is more electronegative than carbon, influencing bond polarity.
- Resonance Structures: The delocalization of electrons in CO3^2- stabilizes the ion and affects bond length.
- Temperature and Pressure: External conditions can alter bond distances in solid-state materials.
Applications of CO3^2- Bond Length Knowledge

Material Science
In material science, understanding CO3^2- bond length is essential for designing carbonates used in construction, ceramics, and polymers. Precise control over bond length can enhance material strength and durability.
Environmental Chemistry
Carbonates play a critical role in carbon sequestration and ocean chemistry. Knowledge of CO3^2- bond length aids in predicting how carbonates interact with atmospheric CO2, impacting climate models.
Pharmaceuticals
In drug development, carbonate ions are often used as intermediates. Understanding their bond length helps optimize reaction pathways for efficient synthesis.
| Application | Relevance of CO3^2- Bond Length |
|---|---|
| Material Science | Enhances material properties like strength and stability. |
| Environmental Chemistry | Improves carbon sequestration and climate modeling. |
| Pharmaceuticals | Optimizes drug synthesis processes. |

How to Measure CO3^2- Bond Length
Measuring CO3^2- bond length involves advanced techniques such as:
- X-ray Crystallography: Provides precise atomic distances in crystalline structures.
- Spectroscopy: Techniques like IR and Raman spectroscopy offer insights into bond vibrations.
- Computational Modeling: Simulations predict bond lengths under various conditions.
📌 Note: Accurate measurement requires high-quality equipment and expertise in analytical chemistry.
Checklist for Studying CO3^2- Bond Length
- Understand Geometry: Familiarize yourself with the trigonal planar structure of CO3^2-.
- Explore Factors: Study how electronegativity, resonance, and external conditions affect bond length.
- Apply Knowledge: Identify real-world applications in material science, environmental chemistry, and pharmaceuticals.
- Use Tools: Learn about X-ray crystallography, spectroscopy, and computational modeling for measurement.
(carbonate ion structure,bond length measurement,chemical bonding)
Understanding CO3^2- bond length is more than a theoretical exercise—it’s a gateway to innovation in multiple fields. By mastering this concept, you can contribute to advancements in materials, environmental science, and beyond.
What is the typical CO3^2- bond length?
+
The typical CO3^2- bond length is approximately 1.30 Å (angstroms) due to its trigonal planar geometry.
How does resonance affect CO3^2- bond length?
+
Resonance delocalizes electrons, stabilizing the ion and resulting in equal bond lengths between carbon and oxygen atoms.
Why is CO3^2- bond length important in environmental chemistry?
+
It helps predict how carbonates interact with CO2, influencing carbon sequestration and ocean chemistry.



