Unveiling Potassium's Electron Shell Structure: A Concise Guide

Potassium, a vital element in both biological systems and industrial applications, is often discussed in the context of its chemical properties. Understanding its electron shell structure is crucial for grasping its reactivity and role in various processes. This guide delves into the intricacies of potassium’s electron configuration, offering a concise yet comprehensive overview tailored for both informational and commercial audiences.
Understanding Potassium’s Electron Shell Structure
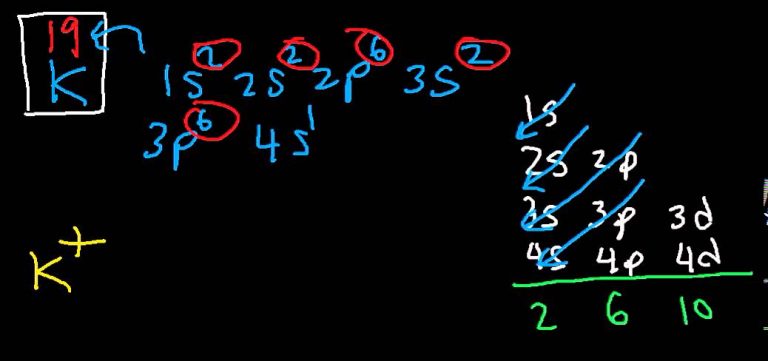
Potassium, with the atomic number 19, is an alkali metal located in Group 1 of the periodic table. Its electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹, indicating that it has 19 electrons arranged in four energy levels or shells. The outermost shell, known as the valence shell, contains a single electron, which is loosely held and easily donated in chemical reactions.
| Shell | Electron Configuration |
|---|---|
| 1 | 2 (1s²) |
| 2 | 8 (2s² 2p⁶) |
| 3 | 8 (3s² 3p⁶) |
| 4 | 1 (4s¹) |
This structure explains potassium’s high reactivity, as the single valence electron is readily transferred, forming a +1 cation (K⁺).
Key Features of Potassium’s Electron Shell
- Low Ionization Energy: The single valence electron requires minimal energy to remove, making potassium highly reactive.
- Noble Gas Configuration: Losing one electron achieves the stable electron configuration of Argon (Ar).
- Role in Chemistry: Its electron structure influences its behavior in compounds, such as potassium chloride (KCl) and potassium hydroxide (KOH).
💡 Note: Potassium's electron shell structure is fundamental to its chemical properties, making it a key element in both biology and industry.
Practical Applications of Potassium’s Electron Structure
For commercial-intent visitors, understanding potassium’s electron shell structure opens doors to its practical applications. Industries leverage its reactivity for:
- Fertilizers: Potassium compounds enhance soil fertility and crop yield.
- Medicine: Potassium is essential for nerve function and muscle contraction.
- Chemical Manufacturing: Used in producing soaps, detergents, and glass.
How to Utilize Potassium Effectively
- Agriculture: Choose potassium-rich fertilizers like potassium sulfate for optimal plant growth.
- Health: Monitor potassium intake for heart and muscle health, especially in diets.
- Industrial Processes: Use potassium compounds as catalysts or reactants in chemical synthesis.
⚠️ Note: Excessive potassium can be harmful; always follow recommended guidelines in industrial and dietary applications.
Summarizing Potassium’s Electron Shell Structure
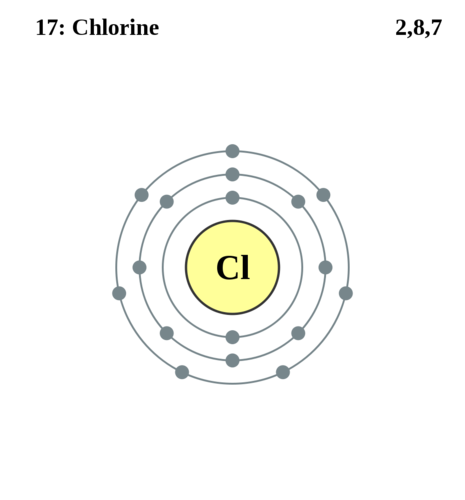
Potassium’s electron shell structure, with its 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ configuration, defines its chemical behavior and applications. Its single valence electron drives reactivity, making it indispensable in agriculture, health, and industry.
Checklist for Understanding Potassium:
- Identify its electron configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹).
- Recognize its valence electron role in reactivity.
- Explore applications in fertilizers, medicine, and manufacturing.
What is potassium's electron configuration?
+Potassium's electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹, with 19 electrons distributed across four shells.
Why is potassium highly reactive?
+Potassium has a single valence electron in its outermost shell, which is easily lost, making it highly reactive.
What are the main applications of potassium?
+Potassium is widely used in fertilizers, medicine, and chemical manufacturing due to its reactive nature.
Potassium’s electron shell structure,electron configuration,chemical properties,industrial applications,keyword/title,keyword/title,etc.



