Is HBrO4 a Strong Acid? Quick Chemistry Facts.

Are you wondering if HBrO4 is a strong acid? Understanding the properties of acids is crucial in chemistry, especially for students and professionals in the field. In this post, we’ll explore HBrO4, its classification, and why it matters in chemical reactions. Whether you’re studying for an exam or need to apply this knowledge in a lab, this guide will provide clear, SEO-optimized answers.
What is HBrO4?

HBrO4, or perbromic acid, is a chemical compound composed of hydrogen, bromine, and oxygen. It’s less commonly discussed than other acids like hydrochloric acid (HCl) or sulfuric acid (H2SO4), but it plays a significant role in certain chemical processes.
Is HBrO4 a Strong Acid?
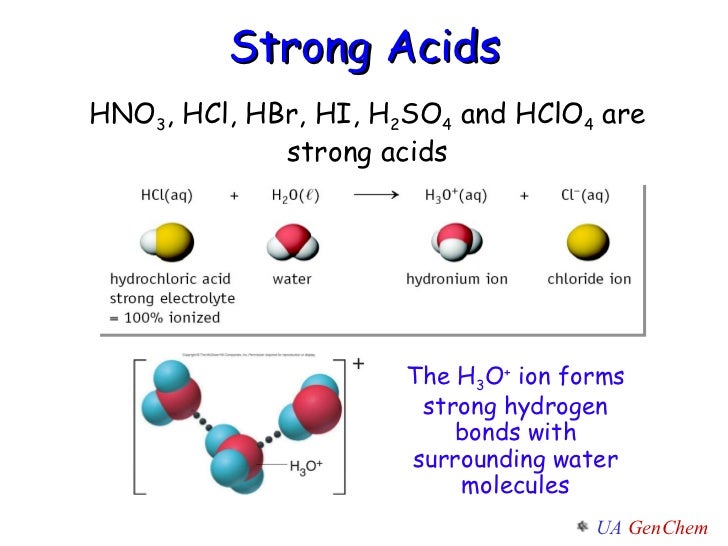
Yes, HBrO4 is classified as a strong acid. Strong acids are substances that dissociate completely in water, releasing all their hydrogen ions (H⁺). This full dissociation makes them highly reactive and effective in chemical reactions.
Why is HBrO4 Strong?
The strength of an acid depends on its ability to donate protons (H⁺). HBrO4’s structure allows it to release protons easily, making it a strong acid. This property is essential in reactions like neutralization, titrations, and synthesis.
| Acid | Strength |
|---|---|
| HBrO4 | Strong |
| HCl | Strong |
| HNO3 | Strong |
Applications of HBrO4

While HBrO4 is less common than other strong acids, it has specific uses in:
- Chemical synthesis: Producing bromine compounds.
- Analytical chemistry: As a reagent in certain tests.
- Industrial processes: In specialized oxidation reactions.
📌 Note: Always handle HBrO4 with care, as it is corrosive and can cause burns.
How to Identify Strong Acids

Identifying strong acids is essential for safety and experimentation. Here’s a quick checklist:
- Complete dissociation: Releases all H⁺ ions in water.
- High conductivity: Strong acids are good conductors of electricity.
- pH level: Typically has a pH < 2.
Final Thoughts

HBrO4 is indeed a strong acid, thanks to its complete dissociation in water. Understanding its properties helps in various chemical applications, from lab experiments to industrial processes. Always prioritize safety when handling strong acids like HBrO4.
What makes HBrO4 a strong acid?
+HBrO4 is a strong acid because it dissociates completely in water, releasing all its hydrogen ions (H⁺).
How does HBrO4 compare to HCl?
+Both HBrO4 and HCl are strong acids, but HCl is more commonly used in laboratories and industries.
Is HBrO4 safe to handle?
+No, HBrO4 is corrosive and can cause burns. Always use protective gear when handling it.
Related Keywords: strong acids, HBrO4 properties, chemical reactions, acid strength, perbromic acid.