Is Melting an Exothermic Process? Explained Simply

Have you ever wondered whether melting is an exothermic process? It’s a common question in chemistry and everyday life, especially when observing ice turning into water or chocolate melting in your hands. Understanding whether melting releases or absorbs heat is crucial for both scientific and practical purposes. In this post, we’ll break down the concept in simple terms, focusing on is melting an exothermic process and its implications.
What is an Exothermic Process?
An exothermic process is one that releases heat energy to its surroundings. Examples include combustion, neutralization reactions, and even the metabolism of food in our bodies. In contrast, an endothermic process absorbs heat from the surroundings. Identifying whether a process is exothermic or endothermic helps us understand energy transfer in chemical and physical changes.
Is Melting Exothermic or Endothermic?
Melting is the process of a solid changing into a liquid, such as ice melting into water. To determine if melting is exothermic, we need to examine the energy flow. During melting, heat energy is absorbed by the substance to break the intermolecular forces holding the particles together. This means melting is not an exothermic process; it is endothermic.
| Process | Heat Flow | Example |
|---|---|---|
| Exothermic | Releases heat | Combustion |
| Endothermic | Absorbs heat | Melting ice |

💡 Note: Melting requires heat input, making it an endothermic process, not exothermic.
Why Does Melting Absorb Heat?

Melting involves breaking the bonds between particles in a solid. These bonds hold the particles in a fixed arrangement. To transition to a liquid state, where particles move more freely, energy is needed to overcome these forces. This energy is absorbed from the surroundings, causing the temperature to drop if no external heat is added.
Practical Examples of Melting as an Endothermic Process
- Ice Melting: When ice melts, it absorbs heat from the environment, which is why it feels cold to the touch.
- Wax Melting: Candles absorb heat to melt the wax, which then vaporizes and burns.
- Chocolate Melting: Chocolate absorbs heat from your hands or a warm room to change from a solid to a liquid state.
Key Takeaways
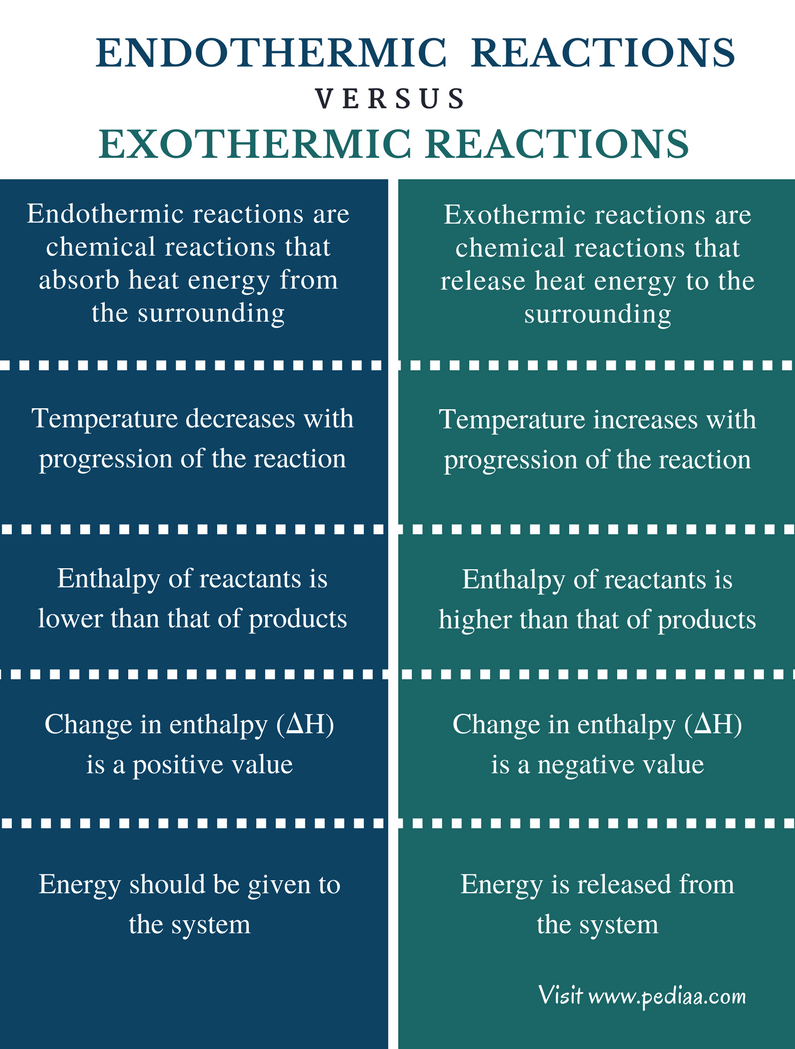
- Melting is an endothermic process, not exothermic, as it absorbs heat.
- Heat is required to break the intermolecular forces in solids.
- Practical examples include ice, wax, and chocolate melting.
Checklist: Understanding Melting
- [ ] Identify whether a process releases or absorbs heat.
- [ ] Recognize that melting absorbs heat, making it endothermic.
- [ ] Apply this knowledge to everyday examples like ice or chocolate melting.
Is melting an exothermic process?
+No, melting is an endothermic process because it absorbs heat from the surroundings.
Why does melting absorb heat?
+Melting absorbs heat to break the intermolecular forces holding particles together in a solid.
What are examples of endothermic processes?
+Examples include melting ice, evaporating water, and photosynthesis.
In summary, melting is not an exothermic process but an endothermic one. It absorbs heat to transform solids into liquids, as seen in everyday examples like ice or chocolate melting. Understanding this distinction helps clarify energy transfer in physical changes, making it a fundamental concept in chemistry and daily life.
exothermic vs endothermic, melting process, heat absorption in melting, endothermic examples, chemistry basics



