Is Sodium Chloride Polar? The Surprising Truth.
Have you ever wondered, "Is sodium chloride polar?" This common household substance, known as table salt, plays a vital role in our daily lives. But its chemical nature often sparks curiosity. In this blog, we’ll explore the polarity of sodium chloride, debunk myths, and provide a clear understanding of its molecular structure. Whether you're a chemistry enthusiast or just curious, this guide will shed light on the surprising truth about sodium chloride’s polarity, (chemical bonding, molecular structure, ionic compounds).
What is Sodium Chloride?
Sodium chloride, chemically represented as NaCl, is an ionic compound formed by the reaction of sodium (Na) and chlorine (Cl). It’s widely recognized as table salt and is essential in food seasoning, medical treatments, and industrial processes. But what makes it unique? Its structure is key to understanding its polarity, (table salt, ionic compound, chemical properties).
Understanding Polarity in Chemistry

Polarity refers to the separation of electric charge in a molecule, creating a positive and negative end. In chemistry, polar molecules have an uneven distribution of charge, while nonpolar molecules have an even distribution. For example, water (H₂O) is polar, but oxygen (O₂) is nonpolar. But where does sodium chloride fit in? (polar molecules, nonpolar molecules, chemical bonds).
Is Sodium Chloride Polar?
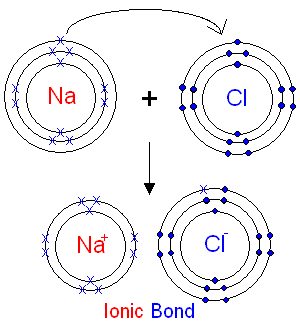
The surprising truth is that sodium chloride is not polar in the traditional sense. Instead, it’s classified as an ionic compound. In NaCl, sodium (a metal) donates an electron to chlorine (a non-metal), forming a strong ionic bond. This bond results in a crystal lattice structure with no distinct molecular polarity. However, individual ions (Na⁺ and Cl⁻) do carry charges, (ionic bond, crystal lattice, molecular polarity).
| Property | Sodium Chloride (NaCl) |
|---|---|
| Type of Bond | Ionic |
| Polarity | Not polar (ionic) |
| Structure | Crystal lattice |

Why Sodium Chloride is Classified as Ionic

Sodium chloride’s ionic nature stems from the complete transfer of electrons between sodium and chlorine. This results in a high melting point, solubility in water, and conductivity in aqueous solutions—all hallmarks of ionic compounds. Unlike polar molecules, NaCl doesn’t have a permanent dipole moment, (ionic compounds, dipole moment, solubility in water).
📌 Note: While sodium chloride is not polar, its ionic nature makes it highly reactive in water, dissolving into Na⁺ and Cl⁻ ions.
Key Takeaways: Sodium Chloride Polarity

- Sodium chloride (NaCl) is an ionic compound, not a polar molecule.
- It forms through the transfer of electrons, creating Na⁺ and Cl⁻ ions.
- Its crystal lattice structure lacks a permanent dipole moment.
- NaCl is soluble in water and conducts electricity when dissolved.
In summary, sodium chloride’s polarity is a common misconception. Its ionic nature, driven by the transfer of electrons, distinguishes it from polar molecules. Understanding this difference is crucial for applications in chemistry, biology, and everyday life. Whether you’re seasoning your food or studying chemical bonds, knowing the truth about NaCl’s structure enhances your appreciation of this essential compound, (chemical bonds, everyday life, essential compound).
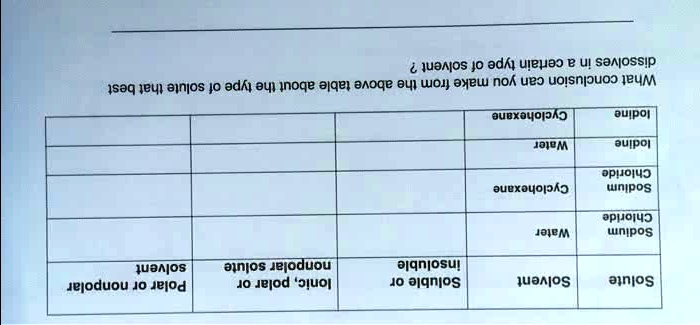
Is sodium chloride polar or nonpolar?
+
Sodium chloride is neither polar nor nonpolar; it’s an ionic compound due to its ionic bonds.
Why is sodium chloride soluble in water?
+
Its ionic nature allows it to dissociate into Na⁺ and Cl⁻ ions, which are attracted to water molecules.
Can sodium chloride conduct electricity?
+
Yes, when dissolved in water or melted, NaCl conducts electricity due to its free-moving ions.



