Lewis Diagram of H2O2: A Visual Guide

Understanding the Lewis diagram of H2O2 is essential for grasping the molecular structure and bonding of hydrogen peroxide. This visual guide breaks down the process step-by-step, making it easy for both students and professionals to comprehend. Whether you're studying chemistry or working in a lab, mastering the H2O2 Lewis structure will enhance your knowledge of chemical compounds. Let’s dive into the details and explore how to draw and interpret this diagram effectively, molecular structure, chemical bonding, hydrogen peroxide.
What is a Lewis Diagram?
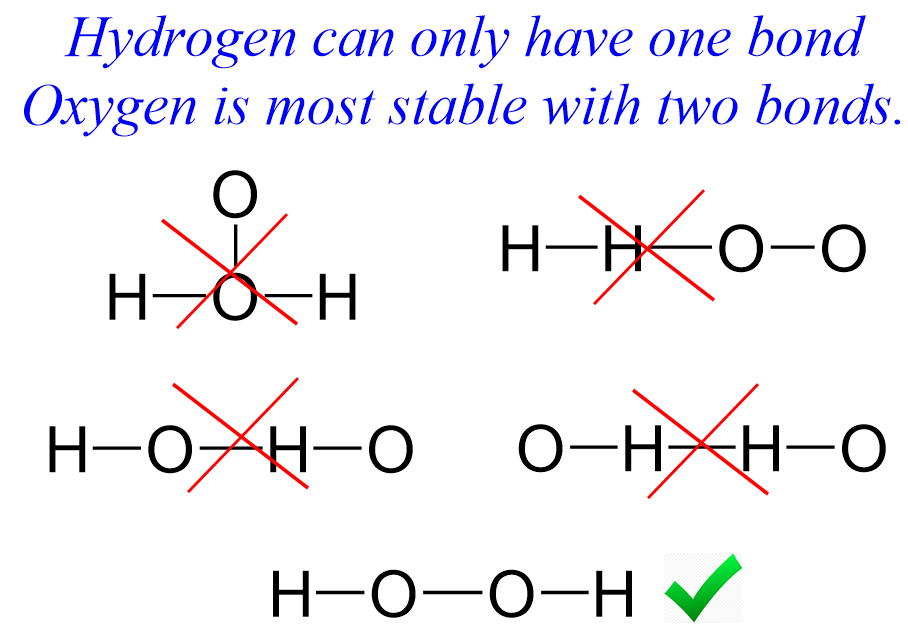
A Lewis diagram, also known as an electron dot diagram, is a visual representation of the distribution of valence electrons in a molecule. It helps predict molecular geometry and understand chemical bonding. For H2O2 (hydrogen peroxide), the Lewis diagram illustrates how oxygen and hydrogen atoms share electrons to form stable bonds, electron dot diagram, molecular geometry, chemical bonding.
Steps to Draw the Lewis Diagram of H2O2

Drawing the H2O2 Lewis structure involves a systematic approach. Follow these steps to create an accurate diagram:
- Step 1: Count the Total Valence Electrons – Hydrogen has 1 electron per atom, and oxygen has 6. For H2O2, calculate: (2 × 1) + (2 × 6) = 14 electrons, valence electrons, hydrogen, oxygen.
- Step 2: Determine the Central Atom – Oxygen is the central atom due to its higher electronegativity, central atom, electronegativity.
- Step 3: Connect Atoms with Single Bonds – Place the two oxygen atoms in the center and connect them with a single bond. Attach hydrogen atoms to each oxygen, single bonds, molecular structure.
- Step 4: Distribute Remaining Electrons – Add lone pairs to oxygen atoms to complete their octets. Each oxygen will have two lone pairs, lone pairs, octet rule.
- Step 5: Check Formal Charges – Ensure the formal charges are minimized for stability, formal charges, molecular stability.
💡 Note: Hydrogen peroxide has a unique structure with an O-O single bond, which is less common in oxygen compounds.
Key Features of the H2O2 Lewis Diagram
The Lewis diagram of H2O2 highlights several important aspects:
- O-O Single Bond – Unlike most oxygen compounds, H2O2 has a single bond between oxygen atoms, O-O bond, molecular structure.
- Lone Pairs on Oxygen – Each oxygen atom has two lone pairs, contributing to its reactivity, lone pairs, chemical reactivity.
- Bent Molecular Geometry – The molecule adopts a bent shape due to lone pair repulsion, molecular geometry, bent shape.
| Component | Details |
|---|---|
| Total Valence Electrons | 14 |
| Central Atom | Oxygen (O) |
| Bonding | O-O single bond, O-H bonds |
| Lone Pairs | 2 per oxygen atom |
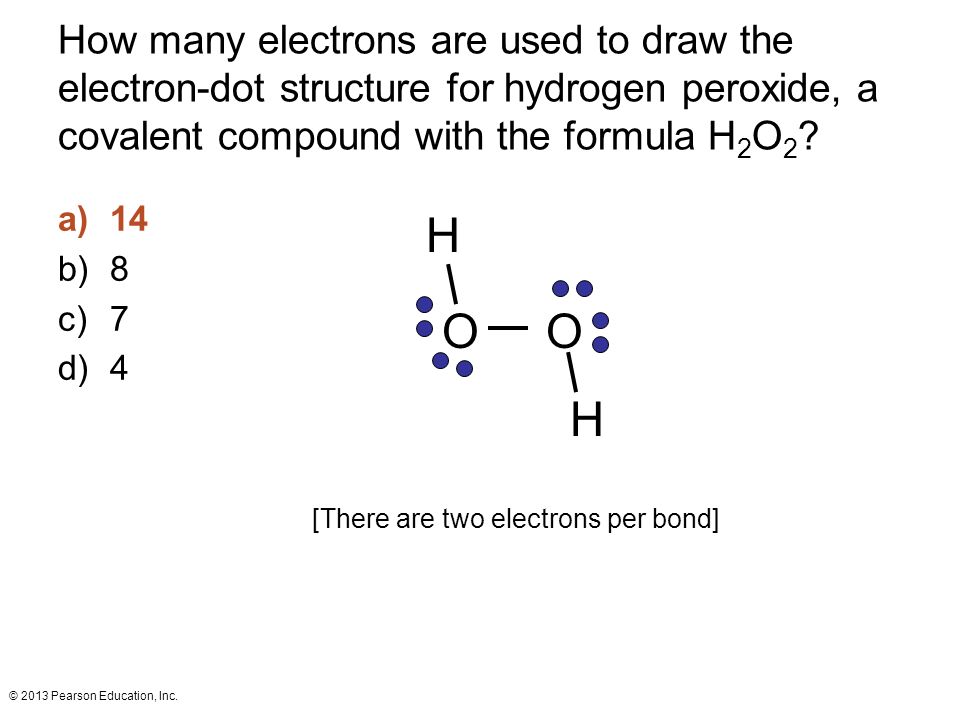
Checklist: Drawing the H2O2 Lewis Diagram
- Count total valence electrons.
- Identify the central atom.
- Connect atoms with single bonds.
- Distribute remaining electrons as lone pairs.
- Check formal charges for stability.
Mastering the Lewis diagram of H2O2 provides valuable insights into its molecular structure and chemical properties. By following the steps outlined in this guide, you can confidently draw and interpret the diagram. Whether for academic or professional purposes, understanding H2O2’s structure is a fundamental skill in chemistry, molecular structure, chemical properties, chemistry fundamentals.
What is the molecular geometry of H2O2?
+
H2O2 has a bent molecular geometry due to the lone pairs on the oxygen atoms, molecular geometry, bent shape.
Why does H2O2 have an O-O single bond?
+
The O-O single bond in H2O2 is due to the molecule’s unique structure, which differs from typical oxygen compounds, O-O bond, molecular structure.
How many lone pairs are on each oxygen atom in H2O2?
+
Each oxygen atom in H2O2 has two lone pairs, contributing to its reactivity, lone pairs, chemical reactivity.



