Mastering BeF2 Lewis Dot Structure: A Quick Guide

Understanding BeF₂ Lewis Dot Structure

BeF₂, or Beryllium Fluoride, is a fascinating molecule with a simple yet crucial Lewis dot structure. Understanding this structure is essential for grasping its chemical properties and behavior. Whether you’re a student studying chemistry or a professional in the field, mastering the BeF₂ Lewis dot structure will enhance your understanding of molecular bonding.
What is a Lewis Dot Structure?
A Lewis dot structure is a diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons that may exist in the molecule. It’s a fundamental concept in chemistry that helps visualize the distribution of electrons in a compound.
Why BeF₂ is Important
Beryllium Fluoride is a key compound in various applications, including nuclear reactors and the manufacturing of ceramics. Its Lewis dot structure provides insights into its linear geometry and nonpolar nature, making it a great starting point for understanding more complex molecules.
Step-by-Step Guide to Drawing BeF₂ Lewis Dot Structure

Step 1: Determine the Total Number of Valence Electrons
- Beryllium (Be) has 2 valence electrons.
- Fluorine (F) has 7 valence electrons, and since there are 2 fluorine atoms, the total is 14.
- Total valence electrons = 2 (Be) + 14 (F) = 16.
Step 2: Identify the Central Atom
In BeF₂, Beryllium (Be) is the central atom because it is less electronegative than fluorine.
Step 3: Connect the Atoms with Single Bonds
Draw single bonds between Beryllium and each Fluorine atom. This uses up 4 electrons (2 bonds × 2 electrons per bond).
Step 4: Complete the Octets
- Each Fluorine atom needs 8 electrons to complete its octet. Since each Fluorine already has 1 bond (2 electrons), add 6 more electrons (3 lone pairs) to each Fluorine atom.
- Beryllium, being in Group 2, does not require a full octet. It will have no lone pairs.
Step 5: Verify the Structure
Ensure all atoms have the correct number of electrons and that the total number of valence electrons (16) is accounted for.
📌 Note: Beryllium is an exception to the octet rule, as it typically forms compounds with only four electrons around it.
Key Properties of BeF₂

Molecular Geometry
BeF₂ has a linear geometry due to the arrangement of its atoms and the absence of lone pairs on the central Beryllium atom.
Polarity
Despite having polar Be-F bonds, BeF₂ is nonpolar overall because the bond dipoles cancel each other out due to the linear geometry.
| Property | Description |
|---|---|
| Molecular Geometry | Linear |
| Polarity | Nonpolar |
| Bond Angle | 180° |
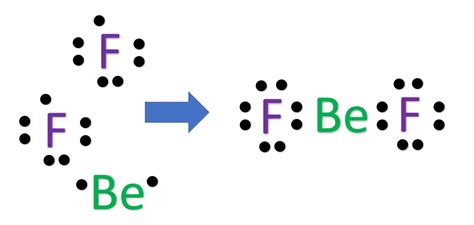
Checklist for Drawing BeF₂ Lewis Dot Structure

- [ ] Count valence electrons: 2 (Be) + 14 (F) = 16.
- [ ] Place Beryllium as the central atom.
- [ ] Draw single bonds between Be and F atoms.
- [ ] Complete Fluorine octets with lone pairs.
- [ ] Verify the total electrons used match the valence electrons.
Wrapping Up
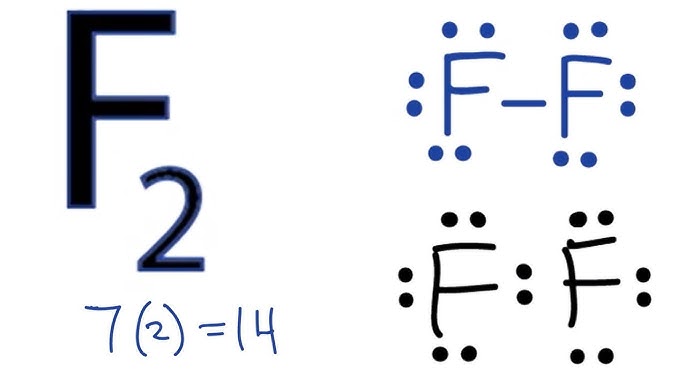
Mastering the BeF₂ Lewis dot structure is a foundational skill in chemistry. By following the steps outlined above, you can confidently draw the structure and understand its molecular properties. Remember, practice is key to becoming proficient in these concepts. Keep exploring and applying these principles to more complex molecules!
What is the molecular geometry of BeF₂?
+BeF₂ has a linear molecular geometry due to the arrangement of its atoms and the absence of lone pairs on the central Beryllium atom.
Is BeF₂ polar or nonpolar?
+BeF₂ is nonpolar because the bond dipoles cancel each other out due to its linear geometry.
Why doesn’t Beryllium follow the octet rule in BeF₂?
+Beryllium, being in Group 2, typically forms compounds with only four electrons around it, making it an exception to the octet rule.
Related Keywords: BeF₂ Lewis structure, molecular geometry, nonpolar molecules, valence electrons, linear geometry, Beryllium Fluoride, chemistry basics, chemical bonding.