How to Draw the Lewis Dot Structure of SCl4
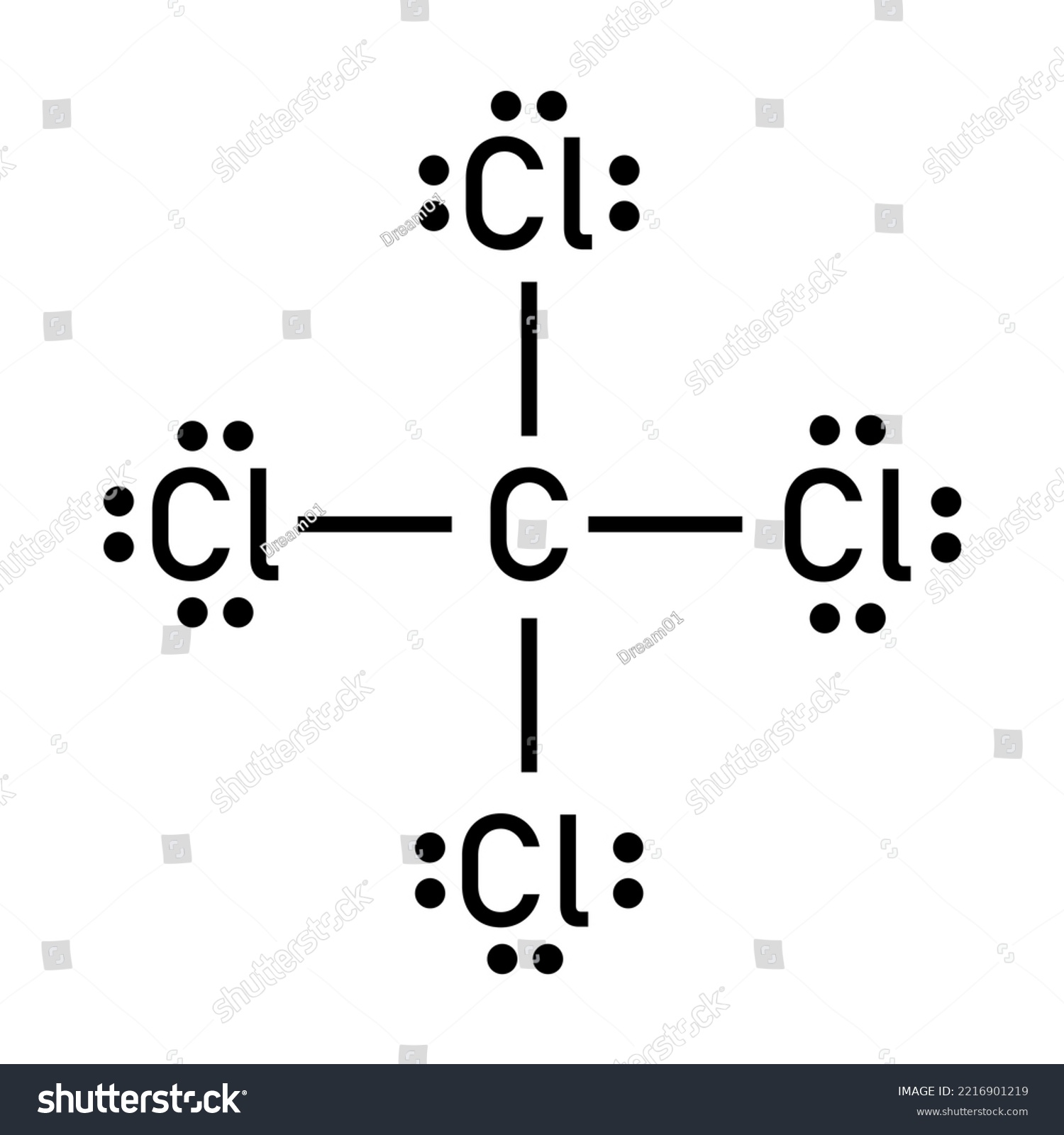
Drawing the Lewis dot structure of SCl4 (sulfur tetrachloride) is a fundamental skill in chemistry, helping you understand the molecule's bonding and electron distribution. This guide provides a step-by-step approach to accurately represent SCl4 using Lewis dot structures, ensuring clarity for both students and professionals. (Lewis dot structure, SCl4, chemical bonding)
Step-by-Step Guide to Drawing the Lewis Dot Structure of SCl4

Step 1: Determine the Total Number of Valence Electrons
Start by identifying the valence electrons of sulfur (S) and chlorine (Cl). Sulfur has 6 valence electrons, and each chlorine atom has 7. Since SCl4 has one sulfur and four chlorine atoms:
- Sulfur: 6 electrons
- Chlorine (4 atoms): 4 × 7 = 28 electrons
- Total: 6 + 28 = 34 valence electrons
Step 2: Identify the Central Atom
Sulfur (S) is the central atom in SCl4 because it is less electronegative than chlorine. (central atom, electronegativity)
Step 3: Connect the Atoms with Single Bonds
Draw single bonds between sulfur and each chlorine atom. This uses up 8 electrons (4 bonds × 2 electrons per bond), leaving 26 electrons for dot placement.
Step 4: Place Remaining Electrons as Lone Pairs
Distribute the remaining 26 electrons as lone pairs on the chlorine atoms, ensuring each chlorine has a complete octet (8 electrons total). Sulfur will have more than 8 electrons, which is acceptable for period 3 elements like sulfur. (octet rule, lone pairs)
📌 Note: Sulfur can accommodate more than 8 electrons due to its expanded octet capability.
Step 5: Verify the Structure
Ensure all atoms (except hydrogen) have a complete octet and that the total number of electrons used matches the calculated valence electrons (34). (formal charge, octet rule)
Checklist for Drawing the Lewis Dot Structure of SCl4

- Calculate total valence electrons: 34
- Place sulfur as the central atom
- Draw single bonds between S and Cl atoms
- Distribute remaining electrons as lone pairs on Cl atoms
- Verify all atoms have a complete octet
Mastering the Lewis dot structure of SCl4 enhances your understanding of molecular geometry and chemical bonding. Practice this method with other compounds to strengthen your chemistry skills. (molecular geometry, chemical bonding)
Why does sulfur have more than 8 electrons in SCl4?
+Sulfur, being in period 3, can accommodate more than 8 electrons due to its expanded octet capability. (expanded octet, sulfur)
How many lone pairs are on the chlorine atoms in SCl4?
+Each chlorine atom has 3 lone pairs, totaling 12 lone pairs in SCl4. (lone pairs, chlorine)
What is the molecular geometry of SCl4?
+SCl4 has a see-saw molecular geometry due to its four bonding pairs and one lone pair on sulfur. (molecular geometry, see-saw)



