lewis structure for chbr3
Understanding the Lewis Structure of CHBr3
Bromoform (CHBr3) is a fascinating molecule with a unique Lewis structure. This structure, a visual representation of the arrangement of atoms and electrons in the molecule, is crucial for understanding its chemical properties and behavior. Let’s delve into the world of Lewis structures and explore the specifics of CHBr3.
Drawing the Lewis Structure: Step-by-Step

To construct the Lewis structure of CHBr3, follow these steps:
Step 1: Count the Total Valence Electrons:
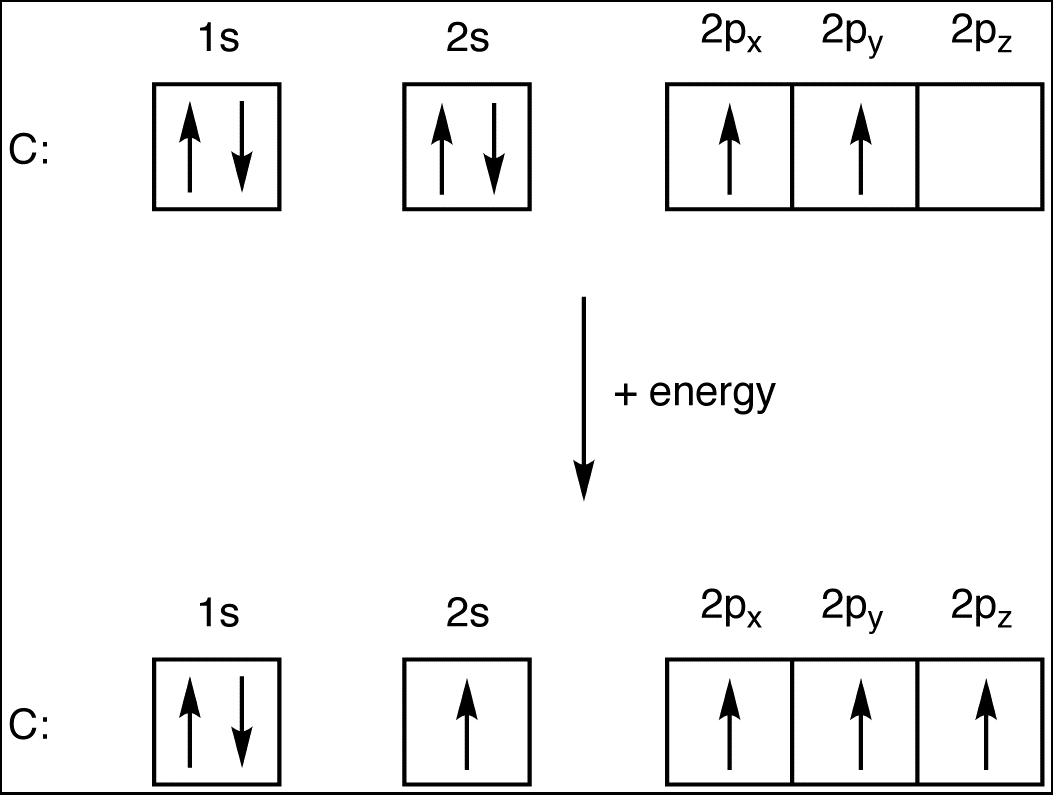
- Carbon © has 4 valence electrons.
- Hydrogen (H) has 1 valence electron.
- Bromine (Br) has 7 valence electrons.
- Total valence electrons = 4 © + 1 (H) + 7 (Br) * 3 = 26.
Step 2: Identify the Central Atom:
Carbon © is the least electronegative atom and typically serves as the central atom in organic compounds.
Step 3: Connect Atoms with Single Bonds:
- Place Carbon in the center and connect it to three Bromine atoms and one Hydrogen atom using single bonds. This uses up 8 valence electrons (4 bonds).
Step 4: Distribute Remaining Electrons:
- Distribute the remaining 18 valence electrons as lone pairs on the Bromine atoms. Each Bromine atom will have 3 lone pairs.
Step 5: Check Octet Rule:
- Carbon has 8 electrons around it (4 from bonds and 4 from lone pairs on Bromine atoms).
- Hydrogen has 2 electrons (from its single bond).
- Each Bromine atom has 8 electrons (2 from the bond and 6 from lone pairs).
📝 Note: Bromine, being in period 4, can accommodate more than 8 electrons due to its d-orbitals, but in CHBr3, it follows the octet rule.
Key Features of CHBr3 Lewis Structure
Tetrahedral Geometry: The molecule adopts a tetrahedral shape due to the arrangement of four electron domains around the central Carbon atom.
Polar Molecule: The presence of highly electronegative Bromine atoms creates a significant electron density difference, making CHBr3 a polar molecule.
Bond Angles: The bond angles around the Carbon atom are approximately 109.5 degrees, characteristic of tetrahedral geometry.
Applications and Significance

Understanding the Lewis structure of CHBr3 is essential for comprehending its: * Solubility: Its polarity influences its solubility in different solvents. * Reactivity: The Lewis structure helps predict how CHBr3 will react with other substances. * Toxicity: Knowledge of its structure contributes to understanding its potential health effects.
Wrapping Up

The Lewis structure of CHBr3 provides a fundamental understanding of its molecular composition and properties. By following the steps outlined above, you can visualize the arrangement of atoms and electrons, paving the way for further exploration of this intriguing molecule’s behavior in various contexts.
What is the molecular geometry of CHBr3?
+CHBr3 has a tetrahedral molecular geometry due to the arrangement of four electron domains around the central Carbon atom.
Is CHBr3 polar or nonpolar?
+CHBr3 is a polar molecule due to the presence of highly electronegative Bromine atoms, creating a significant electron density difference.
What are the bond angles in CHBr3?
+The bond angles in CHBr3 are approximately 109.5 degrees, characteristic of tetrahedral geometry.
What is the total number of valence electrons in CHBr3?
+CHBr3 has a total of 26 valence electrons.
What is the central atom in CHBr3?
+Carbon (C) is the central atom in CHBr3.
lewis structure,molecular geometry,polar molecule,valence electrons,tetrahedral geometry, chemical bonding,electronegativity, organic chemistry,bromoform, CHBr3 structure, CHBr3 properties, CHBr3 polarity, CHBr3 geometry, CHBr3 Lewis dot structure, CHBr3 molecular shape, CHBr3 electron geometry, CHBr3 bond angles, CHBr3 solubility, CHBr3 reactivity, CHBr3 toxicity,