lewis structure of ph3
Understanding the Lewis Structure of PH3: A Comprehensive Guide
Phosphine (PH3) is a colorless, highly toxic gas with a distinctive fish-like odor. Its Lewis structure is essential for understanding its chemical properties, bonding, and reactivity. In this guide, we'll explore the Lewis structure of PH3, its significance, and how to draw it step-by-step. (lewis structure of ph3, phosphine lewis structure, ph3 molecular geometry)
What is a Lewis Structure?

A Lewis structure, also known as an electron dot diagram, is a visual representation of the distribution of valence electrons in a molecule. It helps predict molecular geometry, bond formation, and overall chemical behavior. (lewis dot structure, valence electrons, molecular geometry)
Drawing the Lewis Structure of PH3
Step 1: Determine the Total Number of Valence Electrons
Phosphorus (P) has 5 valence electrons, and each hydrogen (H) atom has 1 valence electron. Since PH3 has one phosphorus and three hydrogen atoms, the total number of valence electrons is:
5 (P) + 3 × 1 (H) = 8 valence electrons
Step 2: Identify the Central Atom
Phosphorus (P) is the central atom, as it is less electronegative than hydrogen. (central atom, electronegativity)
Step 3: Connect Atoms with Single Bonds
Draw single bonds between the central phosphorus atom and the three hydrogen atoms. This uses up 6 valence electrons (3 bonds × 2 electrons per bond).
📌 Note: Each single bond represents 2 shared electrons.
Step 4: Distribute Remaining Electrons
With 2 valence electrons remaining, place them as a lone pair on the phosphorus atom to complete its octet. (lone pair, octet rule)
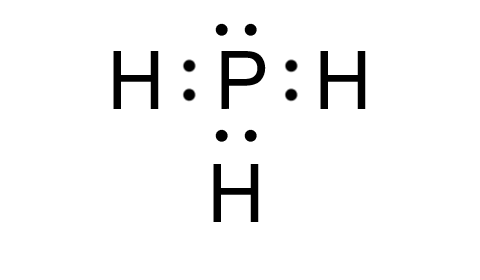
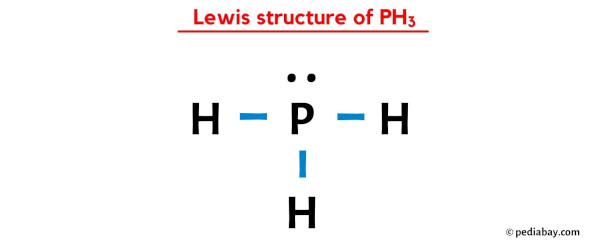
Final Lewis Structure of PH3

The Lewis structure of PH3 consists of a central phosphorus atom bonded to three hydrogen atoms, with one lone pair on phosphorus. This structure reflects its trigonal pyramidal geometry. (trigonal pyramidal, molecular shape)
| Atom | Valence Electrons | Bonds | Lone Pairs |
|---|---|---|---|
| Phosphorus (P) | 5 | 3 | 1 |
| Hydrogen (H) | 1 | 1 | 0 |

Key Takeaways

- PH3 has 8 valence electrons in its Lewis structure.
- The central phosphorus atom forms three single bonds with hydrogen and has one lone pair.
- The molecular geometry of PH3 is trigonal pyramidal.
Mastering the Lewis structure of PH3 is crucial for understanding its chemical behavior and applications. (chemical bonding, phosphine applications)
What is the molecular geometry of PH3?
+
The molecular geometry of PH3 is trigonal pyramidal due to the lone pair on the phosphorus atom.
How many lone pairs are on the phosphorus atom in PH3?
+
There is one lone pair on the phosphorus atom in PH3.
Why is PH3 considered toxic?
+
PH3 is toxic because it inhibits cellular respiration by interfering with oxygen use in cells.


