Molar Mass of C7H6O3: Quick Calculation Guide

Understanding the molar mass of C₇H₆O₃ is essential for students, chemists, and researchers alike. This compound, known as benzoic acid or methyl furan-2-carboxylate, plays a crucial role in various chemical processes and industries. Whether you’re preparing for an exam or working in a lab, knowing how to calculate its molar mass accurately is a fundamental skill. In this guide, we’ll walk you through the quick calculation steps, provide informative insights, and offer practical tips to ensure you master this topic.
What is the Molar Mass of C₇H₆O₃?
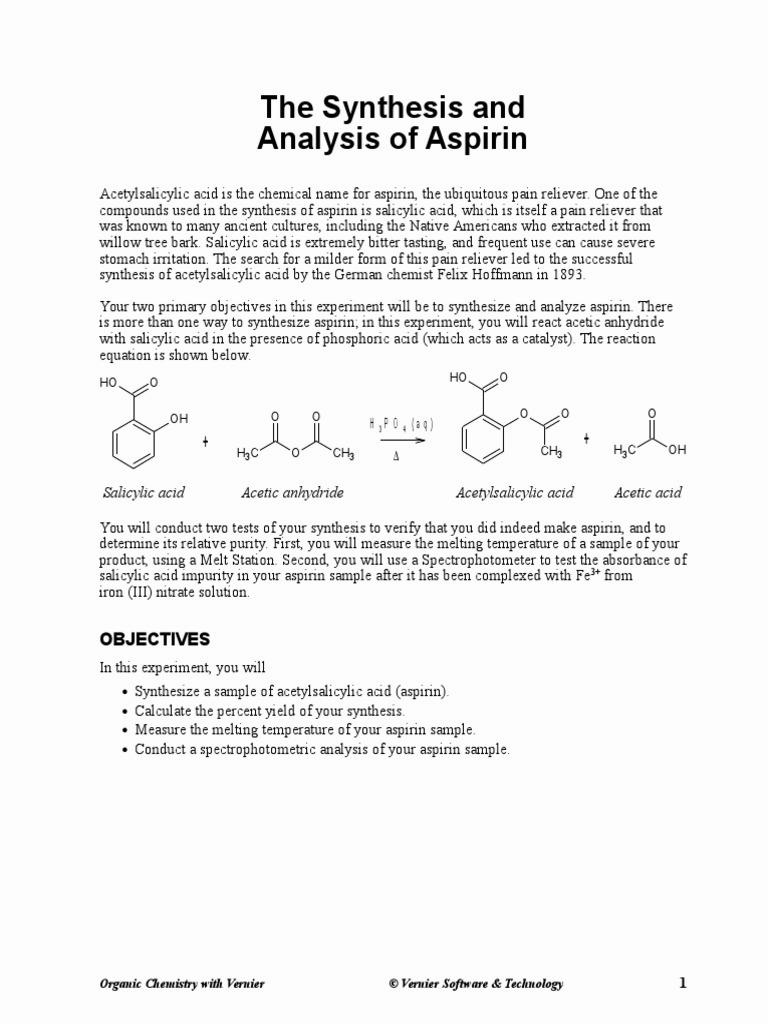
The molar mass of a compound is the sum of the atomic masses of all atoms in its molecular formula. For C₇H₦O₃, it’s calculated by adding the atomic masses of carbon ©, hydrogen (H), and oxygen (O) based on their respective counts in the formula.
Step-by-Step Calculation
Identify Atomic Masses:
- Carbon ©: 12.01 g/mol
- Hydrogen (H): 1.01 g/mol
- Oxygen (O): 16.00 g/mol
- Carbon ©: 12.01 g/mol
Multiply by Atom Count:
- Carbon: 7 atoms × 12.01 g/mol = 84.07 g/mol
- Hydrogen: 6 atoms × 1.01 g/mol = 6.06 g/mol
- Oxygen: 3 atoms × 16.00 g/mol = 48.00 g/mol
- Carbon: 7 atoms × 12.01 g/mol = 84.07 g/mol
Sum the Values:
Total molar mass = 84.07 + 6.06 + 48.00 = 138.13 g/mol
📌 Note: Always use the most accurate atomic masses from the periodic table for precise calculations.
Why is Molar Mass Important?

The molar mass of C₇H₆O₃ is crucial for:
- Stoichiometry: Balancing chemical equations and determining reactant/product ratios.
- Solution Preparation: Calculating the amount of substance needed for a specific concentration.
- Industrial Applications: Used in food preservation, pharmaceuticals, and organic synthesis.
Practical Tips for Accurate Calculation

- Double-Check Atomic Masses: Ensure you’re using the latest values from reliable sources.
- Use a Calculator: Minimize errors by using a scientific calculator or online molar mass calculator.
- Practice Regularly: Familiarize yourself with common compounds to improve speed and accuracy.
Molar Mass of C₇H₆O₃: Checklist

- [ ] Identify the molecular formula: C₇H₆O₃.
- [ ] Gather atomic masses: C (12.01 g/mol), H (1.01 g/mol), O (16.00 g/mol).
- [ ] Multiply atomic masses by atom counts.
- [ ] Sum the results for the total molar mass.
- [ ] Verify your calculation using an online tool or periodic table.
The molar mass of C₇H₆O₃ is 138.13 g/mol, a value critical for various chemical applications. By following the steps outlined above, you can confidently calculate molar masses for any compound. Remember, accuracy is key—always double-check your atomic masses and calculations. Whether you’re a student or a professional, mastering this skill will enhance your chemical expertise.
What is the molar mass of C₇H₆O₃?
+The molar mass of C₇H₆O₃ is 138.13 g/mol.
Why is molar mass important in chemistry?
+Molar mass is crucial for stoichiometry, solution preparation, and understanding chemical reactions.
How do I calculate the molar mass of a compound?
+Multiply the atomic mass of each element by its atom count in the formula and sum the results.
Related Keywords: molar mass calculation, C₇H₆O₃ formula, benzoic acid molar mass, chemical calculations, stoichiometry guide.



