Molar Mass of Potassium Hydroxide: Quick Guide

Understanding the molar mass of potassium hydroxide (KOH) is essential for various applications, from chemical experiments to industrial processes. Potassium hydroxide, a strong base, plays a crucial role in industries like soap making, battery production, and water treatment. Knowing its molar mass helps in precise measurements and reactions. Let’s dive into the details to simplify this concept for both informational and commercial purposes.
What is the Molar Mass of Potassium Hydroxide?

The molar mass of KOH is the sum of the atomic masses of its constituent elements: potassium (K), oxygen (O), and hydrogen (H). Using the periodic table, we find:
- Potassium (K): 39.10 g/mol
- Oxygen (O): 16.00 g/mol
- Hydrogen (H): 1.01 g/mol
Adding these values together:
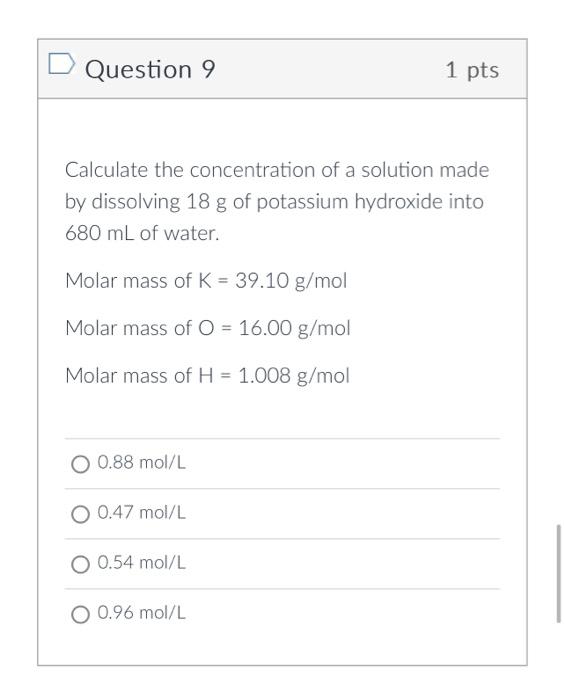
Molar Mass of KOH = 39.10 + 16.00 + 1.01 = 56.11 g/mol
📌 Note: Always ensure accurate atomic masses for precise calculations.
How to Calculate Molar Mass: Step-by-Step Guide

- Identify the Chemical Formula: Potassium hydroxide is represented as KOH.
- Find Atomic Masses: Use the periodic table to locate the atomic masses of K, O, and H.
- Sum the Masses: Add the atomic masses of all elements in the formula.
| Element | Atomic Mass (g/mol) |
|---|---|
| Potassium (K) | 39.10 |
| Oxygen (O) | 16.00 |
| Hydrogen (H) | 1.01 |
| Total (KOH) | 56.11 |

Applications of Potassium Hydroxide

Potassium hydroxide is widely used in:
- Soap and Detergent Manufacturing: Acts as a catalyst in saponification.
- Batteries: Used in alkaline batteries as an electrolyte.
- Water Treatment: Helps neutralize acidic water.
For commercial purposes, understanding the molar mass of KOH ensures consistent product quality and efficiency in industrial processes.
Quick Checklist for Molar Mass Calculation

- Verify the chemical formula: KOH.
- Use accurate atomic masses from the periodic table.
- Double-check the final molar mass: 56.11 g/mol.
Final Thoughts
Mastering the molar mass of potassium hydroxide is fundamental for both academic and industrial applications. Whether you’re a student, researcher, or industry professional, this quick guide simplifies the process and ensures accuracy in your calculations.
What is the molar mass of KOH?
+The molar mass of potassium hydroxide (KOH) is 56.11 g/mol.
Why is knowing the molar mass of KOH important?
+It ensures accurate measurements in chemical reactions and industrial processes, such as soap making and battery production.
How do I calculate the molar mass of KOH?
+Add the atomic masses of potassium (39.10 g/mol), oxygen (16.00 g/mol), and hydrogen (1.01 g/mol) to get 56.11 g/mol.
molar mass of potassium hydroxide, potassium hydroxide uses, chemical calculations, industrial applications, soap making, battery production, water treatment.