Master Balancing Equations with Practice Worksheet Answers
<!DOCTYPE html>
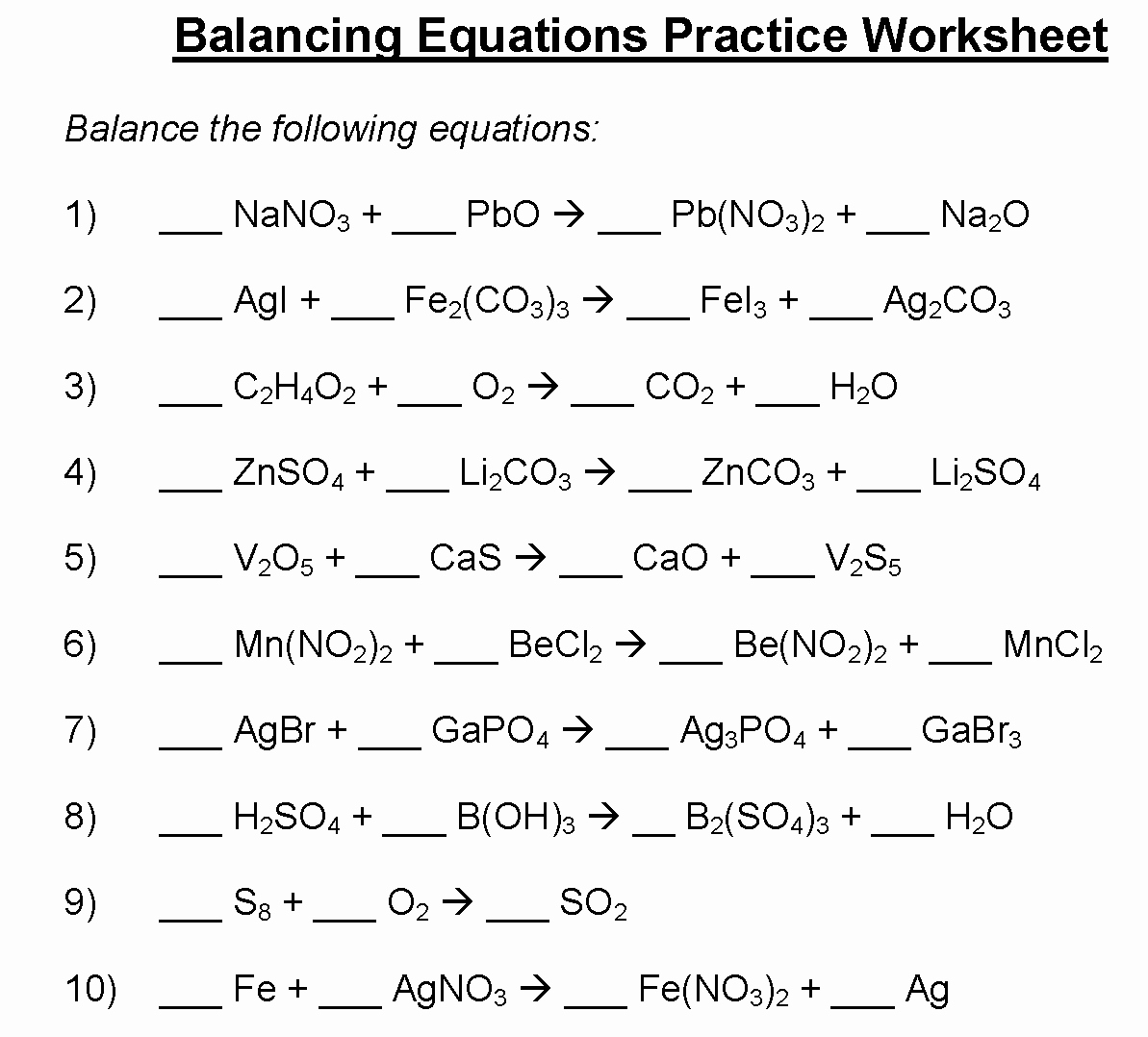
Balancing chemical equations is a fundamental skill in chemistry that ensures the law of conservation of mass is upheld. Whether you’re a student or a professional, mastering this skill is crucial for success in the field. With the right practice and resources, such as balancing equations practice worksheet answers, you can enhance your understanding and confidence in solving complex problems. This guide will walk you through the essentials, providing tips, strategies, and insights to help you excel.
Why Balancing Equations is Essential

Balancing chemical equations is not just about following rules; it’s about understanding the principles of chemistry. It ensures that the number of atoms of each element is the same on both sides of the equation, reflecting real-world chemical reactions. Practice worksheets with answers are invaluable tools for reinforcing this skill, allowing you to check your work and identify areas for improvement.
Step-by-Step Guide to Balancing Equations

1. Identify the Elements and Their Atoms
Start by listing the elements involved in the reaction and counting the number of atoms for each element on both sides of the equation. This initial step sets the foundation for balancing.
2. Begin with the Most Complex Molecule
Balance the equation by starting with the molecule that has the most elements or the one that appears least frequently. This approach simplifies the process and reduces the risk of errors.
3. Balance One Element at a Time
Focus on balancing one element at a time, using coefficients to adjust the number of atoms. Avoid altering the subscripts, as this changes the chemical identity of the compound.
4. Check and Double-Check
Once you’ve balanced all elements, double-check your work to ensure accuracy. Practice worksheets with answers are perfect for this, as they allow you to verify your solutions and learn from mistakes.
📌 Note: Always ensure the final equation has the same number of atoms for each element on both sides.
Benefits of Using Practice Worksheets

Practice worksheets are an excellent resource for honing your balancing skills. They provide a structured way to practice, with answers included to help you assess your progress. Here are some key benefits:
- Immediate Feedback: Check your answers instantly to identify mistakes.
- Varied Problems: Encounter different types of equations to build versatility.
- Confidence Building: Consistent practice boosts your confidence in tackling complex problems.
Tips for Effective Practice

To make the most of your practice sessions, consider these tips:
- Set a Schedule: Dedicate regular time to practice balancing equations.
- Start Simple: Begin with basic equations and gradually move to more complex ones.
- Use Resources: Leverage practice worksheets with answers and online tutorials for guidance.
Checklist for Balancing Equations

Use this checklist to ensure you’re on the right track:
- Identify all elements and count atoms on both sides.
- Balance one element at a time, starting with the most complex molecule.
- Avoid altering subscripts; use coefficients instead.
- Double-check your work for accuracy.
By following these steps and utilizing balancing equations practice worksheet answers, you’ll become proficient in balancing chemical equations. Consistent practice and the right resources are key to mastering this essential chemistry skill. balancing equations practice worksheet answers, chemical equation balancing, chemistry practice worksheets.
What is the purpose of balancing chemical equations?
+Balancing chemical equations ensures the law of conservation of mass is upheld by having the same number of atoms for each element on both sides of the equation.
How do practice worksheets help in learning?
+Practice worksheets provide structured problems with answers, allowing you to practice, check your work, and identify areas for improvement.
Can I alter subscripts while balancing equations?
+No, altering subscripts changes the chemical identity of the compound. Use coefficients to balance the equation instead.