Rutherford-Bohr Model Explained: Atom Structure Simplified

Understanding the structure of an atom is fundamental to grasping the basics of chemistry and physics. The Rutherford-Bohr Model simplifies this complex concept, making it easier to visualize how atoms are organized. This model, a blend of Ernest Rutherford's and Niels Bohr's theories, revolutionized our understanding of atomic structure. Whether you're a student, educator, or simply curious about science, this guide will break down the Rutherford-Bohr Model into digestible parts, ensuring you grasp its core principles. (atom structure, atomic models, Rutherford-Bohr Model)
What is the Rutherford-Bohr Model?
The Rutherford-Bohr Model is a depiction of the atom that combines Rutherford's nuclear model with Bohr's quantum theory. It describes the atom as a small, positively charged nucleus surrounded by electrons orbiting in specific energy levels or shells. This model introduced the concept of quantization, explaining why electrons do not crash into the nucleus. (atomic theory, electron orbits, quantization)
Key Components of the Rutherford-Bohr Model

1. Nucleus
The nucleus is the central part of the atom, containing protons and neutrons. It holds most of the atom’s mass and carries a positive charge. Rutherford’s gold foil experiment confirmed the existence of the nucleus, proving it to be dense and positively charged. (nucleus, protons, neutrons)
2. Electron Orbits (Energy Levels)
Electrons in the Rutherford-Bohr Model occupy specific energy levels or shells around the nucleus. These orbits are quantized, meaning electrons can only exist at certain distances from the nucleus. Bohr’s theory explained that electrons do not radiate energy as long as they remain in these fixed orbits. (energy levels, electron shells, quantization)
3. Quantization of Energy
One of the most groundbreaking aspects of the Rutherford-Bohr Model is the quantization of energy. Electrons can only have specific energy values, and they transition between orbits by absorbing or emitting energy. This concept laid the foundation for quantum mechanics. (quantization, energy transitions, quantum mechanics)
| Component | Description |
|---|---|
| Nucleus | Central core containing protons and neutrons. |
| Electron Orbits | Fixed energy levels where electrons reside. |
| Quantization | Electrons have specific, discrete energy values. |

Limitations of the Rutherford-Bohr Model

While the Rutherford-Bohr Model was a significant advancement, it has limitations. It fails to explain the behavior of atoms with more than one electron and does not account for the wave-particle duality of electrons. These shortcomings led to the development of more advanced models like the Quantum Mechanical Model. (limitations, wave-particle duality, Quantum Mechanical Model)
💡 Note: The Rutherford-Bohr Model is best for understanding simple atoms like hydrogen but is less effective for complex atoms.
Summary and Key Takeaways
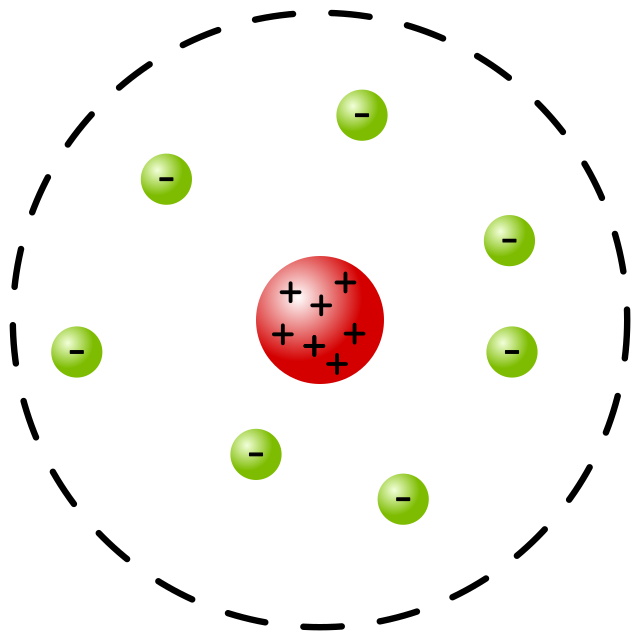
The Rutherford-Bohr Model simplifies atomic structure by depicting a nucleus surrounded by quantized electron orbits. It introduced the concept of energy quantization, which was revolutionary for its time. However, it has limitations, especially for complex atoms. (atomic structure, energy quantization, limitations)
Checklist: Understanding the Rutherford-Bohr Model
- Know the components: nucleus, electron orbits, and quantization.
- Understand how electrons transition between energy levels.
- Recognize the model’s limitations for complex atoms.
In summary, the Rutherford-Bohr Model is an essential stepping stone in our understanding of atomic structure. It bridges classical and quantum physics, providing a clear, if simplified, picture of the atom. While it has been surpassed by more advanced theories, its principles remain foundational in chemistry and physics education. (atomic structure, quantum physics, chemistry education)
What is the main difference between Rutherford’s and Bohr’s models?
+
Rutherford’s model features a nucleus with electrons orbiting randomly, while Bohr’s model introduces quantized orbits and energy levels.
Why is the Rutherford-Bohr Model important?
+
It laid the groundwork for quantum mechanics by introducing the concept of quantized energy levels in atoms.
What are the limitations of the Rutherford-Bohr Model?
+
It cannot explain the behavior of multi-electron atoms or the wave-particle duality of electrons.



