Understanding SCN Resonance Structures: A Simple Guide
Understanding SCN Resonance Structures: A Simple Guide
If you're studying chemistry, you've likely encountered the concept of SCN resonance structures. These structures are crucial in understanding the behavior of molecules, particularly in chemical reactions. In this guide, we'll break down the basics of SCN resonance structures, their importance, and how to draw them. Whether you're a student or a professional, this post will provide valuable insights into chemical bonding, molecular geometry, and resonance theory.
What are SCN Resonance Structures?

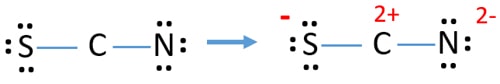
SCN, or thiocyanate, is a polyatomic ion with the formula SCN-. Its resonance structures are different representations of the ion's electron arrangement. These structures help illustrate how electrons are distributed among the atoms, which is essential for predicting reactivity and stability. Resonance structures are particularly important for ions like SCN, as they exhibit delocalized electrons, meaning electrons are shared among multiple atoms.
Why are SCN Resonance Structures Important?

Understanding SCN resonance structures is vital for several reasons. First, they provide insights into the electronic structure of the ion, which influences its chemical properties. Second, resonance structures help explain the ion's reactivity in various chemical reactions. By mastering these concepts, you'll be better equipped to tackle complex problems in inorganic chemistry, organic chemistry, and analytical chemistry.
How to Draw SCN Resonance Structures

Drawing SCN resonance structures involves the following steps:
- Identify the central atom (usually sulfur in SCN)
- Arrange the surrounding atoms (carbon and nitrogen) around the central atom
- Distribute electrons to satisfy the octet rule
- Draw double-headed arrows between resonance structures to indicate electron delocalization
📝 Note: Ensure all resonance structures have the same number of electrons and satisfy the octet rule for each atom.
Example: SCN- Resonance Structures
Here’s a simple example of SCN- resonance structures:
| Structure | Electron Distribution |
|---|---|
| S-C≡N | Electrons localized on specific atoms |
| S=C=N | Electrons delocalized among atoms |
Key Takeaways

To summarize, understanding SCN resonance structures involves grasping the concepts of electron delocalization, resonance theory, and chemical bonding. By following the steps outlined above, you can effectively draw and analyze these structures. Remember to:
- Identify the central atom and surrounding atoms
- Satisfy the octet rule for each atom
- Use double-headed arrows to indicate resonance
With this knowledge, you'll be well-prepared to tackle problems related to SCN resonance structures in chemistry exams, research projects, or professional applications, (resonance structures, chemical bonding, molecular geometry, etc.)
What is the importance of resonance structures in chemistry?
+Resonance structures help explain the stability, reactivity, and electronic distribution of molecules and ions, making them essential in understanding chemical behavior.
How many resonance structures does SCN- have?
+SCN- typically has two main resonance structures, but understanding the concept of resonance is more important than counting structures.
Can SCN- form coordinate covalent bonds?
+Yes, SCN- can act as a ligand and form coordinate covalent bonds with metal ions, which is common in coordination chemistry.


