Simple Diffusion Explained: How It Works

Simple diffusion is a fundamental process in biology and chemistry, allowing molecules to move from an area of high concentration to an area of low concentration. This passive transport mechanism requires no energy and is essential for various natural phenomena, from gas exchange in lungs to nutrient absorption in cells. Understanding simple diffusion is crucial for students, researchers, and professionals in fields like biology, chemistry, and environmental science. In this post, we’ll explore how simple diffusion works, its factors, and real-world applications, all while optimizing for SEO and reader engagement. (Simple Diffusion, Passive Transport, Molecular Movement)
What is Simple Diffusion?
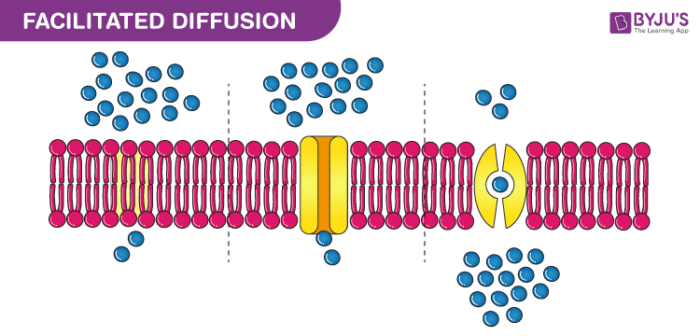
Simple diffusion is the spontaneous movement of particles across a semipermeable membrane. Unlike active transport, it doesn't require energy or specific proteins. Instead, it relies on the natural tendency of molecules to distribute evenly in a given space. This process is driven by the concentration gradient, where molecules move from higher to lower concentrations until equilibrium is reached. (Concentration Gradient, Semipermeable Membrane, Equilibrium)
How Does Simple Diffusion Work?
The Role of Kinetic Energy
Molecules in a solution are constantly in motion due to their kinetic energy. In simple diffusion, this energy allows them to collide with the membrane and pass through if the membrane is permeable to the substance. Smaller, non-polar molecules like oxygen and carbon dioxide diffuse more easily than larger or polar ones. (Kinetic Energy, Molecular Size, Membrane Permeability)
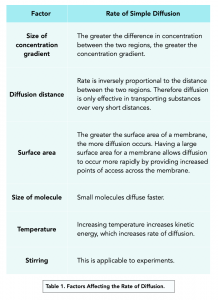
Factors Affecting Simple Diffusion
Several factors influence the rate of simple diffusion:
- Concentration Gradient: A steeper gradient increases diffusion speed.
- Temperature: Higher temperatures enhance molecular movement.
- Molecular Weight: Lighter molecules diffuse faster.
- Membrane Thickness: Thinner membranes allow quicker diffusion.
Real-World Applications of Simple Diffusion
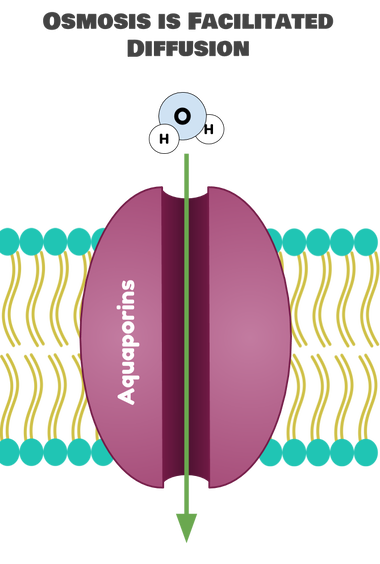
Simple diffusion plays a vital role in biological systems and industrial processes. For instance, it enables oxygen to enter cells and carbon dioxide to exit during respiration. In industries, it’s used in processes like water purification and gas separation. Understanding these applications can help optimize efficiency and innovation. (Biological Systems, Industrial Processes, Gas Exchange)
📌 Note: Simple diffusion is not effective for large molecules or those requiring energy-driven transport, where facilitated diffusion or active transport mechanisms are needed.
In summary, simple diffusion is a passive process driven by concentration gradients and molecular motion. Key factors like temperature, molecular size, and membrane properties influence its rate. From biological functions to industrial applications, understanding simple diffusion is essential for various fields. By grasping these concepts, you can better appreciate the natural and engineered systems around us. (Passive Transport, Concentration Gradient, Molecular Motion)
What is the difference between simple diffusion and facilitated diffusion?
+Simple diffusion requires no transport proteins and works for small, non-polar molecules, while facilitated diffusion uses proteins to transport larger or polar molecules across the membrane.
Can simple diffusion occur against a concentration gradient?
+No, simple diffusion only occurs along the concentration gradient, from high to low concentration. Movement against the gradient requires energy, as in active transport.
Why is simple diffusion important in the lungs?
+In the lungs, simple diffusion allows oxygen to move from inhaled air into the bloodstream and carbon dioxide to move from the blood into the lungs for exhalation.


