t-Butyl Chloride: Properties, Uses, and Safety Tips
Opening Paragraph
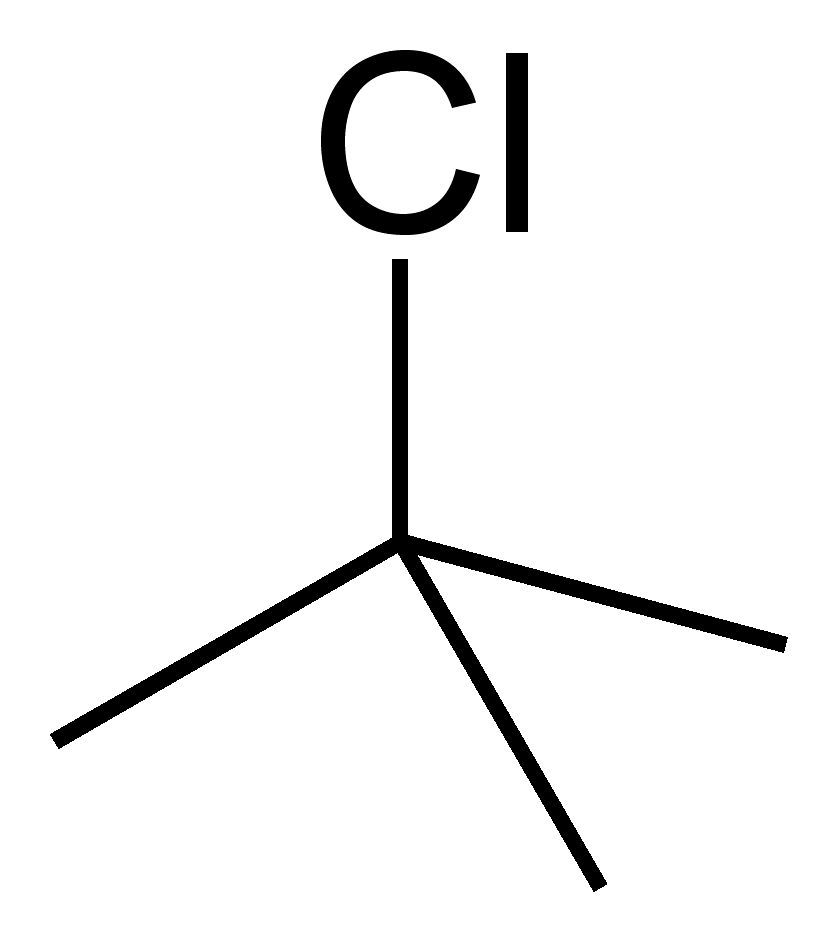
T-Butyl chloride, also known as tert-butyl chloride or 2-chloro-2-methylpropane, is a versatile organic compound widely used in chemical synthesis and industrial applications. Its unique structure, featuring a chlorine atom attached to a tertiary carbon, makes it a valuable reagent in organic chemistry. This blog explores the properties of t-butyl chloride, its uses in various industries, and essential safety tips for handling this compound effectively. Whether you’re a chemist, researcher, or industry professional, understanding t-butyl chloride is crucial for optimizing its applications while ensuring safety.
Properties of T-Butyl Chloride

T-Butyl chloride is a colorless liquid with a characteristic chloroform-like odor. Its chemical formula is (CH₃)₃CCl, and it belongs to the class of alkyl halides. Key properties include:
- Boiling Point: 51°C (124°F)
- Density: 0.87 g/cm³
- Solubility: Insoluble in water but soluble in organic solvents like ethanol and ether.
| Property | Value |
|---|---|
| Molecular Weight | 92.59 g/mol |
| Melting Point | -90°C (-130°F) |
| Refractive Index | 1.395 |

📌 Note: T-Butyl chloride is highly reactive due to its tertiary carbon, making it a useful intermediate in organic synthesis.
Uses of T-Butyl Chloride

T-Butyl chloride is employed in various industries, including pharmaceuticals, agriculture, and polymer production. Its primary applications include:
- Chemical Synthesis: Used as an alkylating agent to introduce tert-butyl groups into molecules.
- Pharmaceuticals: Serves as a building block for synthesizing drugs and intermediates.
- Agriculture: Acts as a precursor for herbicides and pesticides.
- Polymer Industry: Utilized in the production of specialty polymers and resins.
For commercial-intent visitors, t-butyl chloride is available in bulk quantities from chemical suppliers, ensuring high purity and competitive pricing for industrial applications.
Safety Tips for Handling T-Butyl Chloride

While t-butyl chloride is a valuable compound, it poses health and safety risks if mishandled. Follow these precautions:
- Personal Protective Equipment (PPE): Wear gloves, goggles, and lab coats to avoid skin and eye contact.
- Ventilation: Work in a fume hood or well-ventilated area to prevent inhalation of vapors.
- Storage: Store in a cool, dry place away from heat, flames, and oxidizing agents.
- Disposal: Dispose of t-butyl chloride according to local regulations to prevent environmental contamination.
⚠️ Note: T-butyl chloride is flammable and can release toxic hydrogen chloride gas upon decomposition. Handle with care.
Final Thoughts
T-Butyl chloride is a powerful reagent with diverse applications in chemistry and industry. By understanding its properties, uses, and safety precautions, you can maximize its potential while minimizing risks. Whether for research or commercial purposes, proper handling and storage are essential for working with this compound effectively.
Checklist for Safe Handling of T-Butyl Chloride
- [ ] Wear appropriate PPE (gloves, goggles, lab coat).
- [ ] Ensure proper ventilation or use a fume hood.
- [ ] Store in a cool, dry place away from heat sources.
- [ ] Follow local regulations for disposal.
Related Keywords: t-butyl chloride properties, uses of t-butyl chloride, t-butyl chloride safety, alkyl halides, organic synthesis, chemical reagents.
What is t-butyl chloride used for?
+
T-butyl chloride is used in chemical synthesis, pharmaceuticals, agriculture, and polymer production as an alkylating agent and precursor.
Is t-butyl chloride dangerous?
+
Yes, it is flammable, can release toxic gases, and causes skin and eye irritation. Proper safety measures are essential.
How should t-butyl chloride be stored?
+
Store in a cool, dry place away from heat, flames, and oxidizing agents in tightly sealed containers.


