Methane's Boiling Point: A Quick Guide
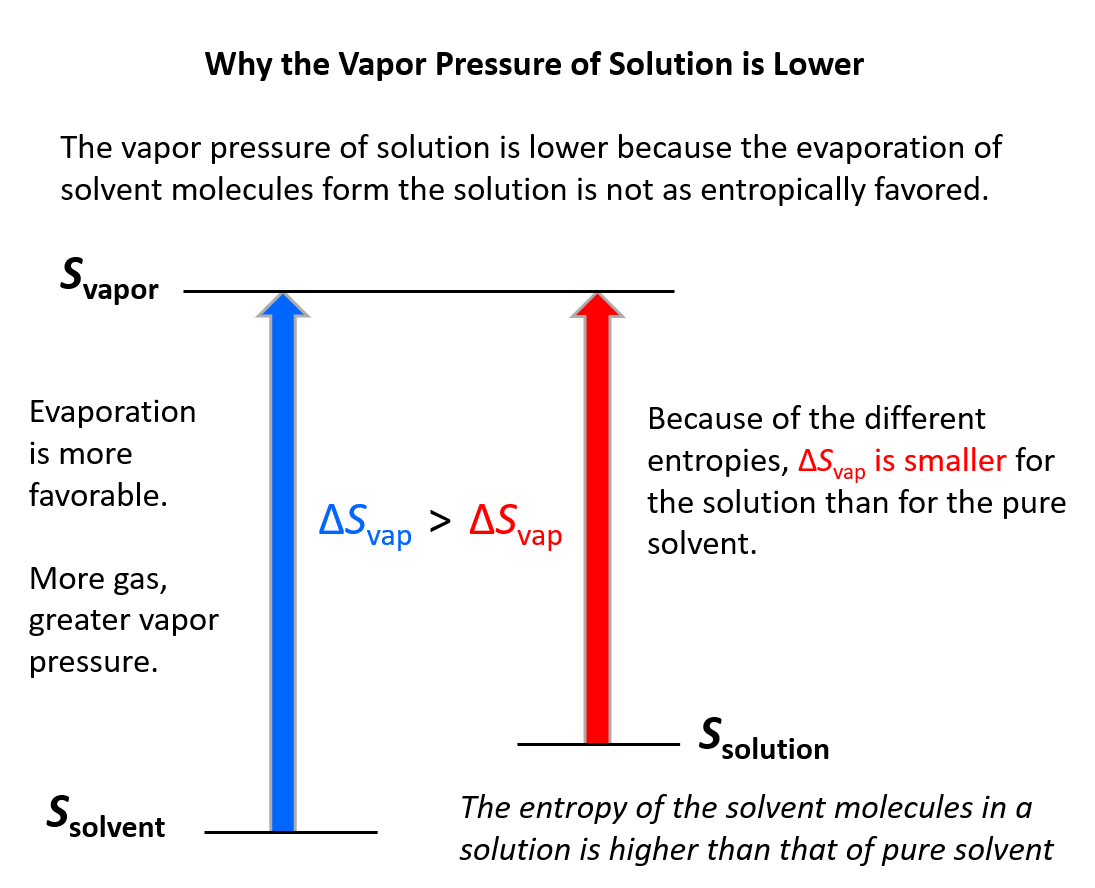
Methane, a key component of natural gas, plays a significant role in various industries, from energy production to chemical manufacturing. Understanding its boiling point is crucial for safe handling, storage, and application. This guide delves into methane’s boiling point, its significance, and practical considerations for both informational and commercial purposes.
What is Methane’s Boiling Point?

Methane (CH₄) has a boiling point of -161.5°C (-258.7°F) at standard atmospheric pressure. This low boiling point makes methane a cryogenic gas, meaning it exists as a gas at room temperature and must be stored or transported under specific conditions to remain liquefied.
💡 Note: Methane's boiling point is critical for industries like liquefied natural gas (LNG) production, where it ensures efficient storage and transportation.
Why Does Methane’s Boiling Point Matter?

Industrial Applications
Methane’s boiling point is vital for processes such as LNG production, refrigeration, and chemical synthesis. It determines the energy required to liquefy methane and the conditions needed to keep it in a liquid state.
Environmental Impact
Understanding methane’s boiling point helps in managing greenhouse gas emissions. Methane is a potent greenhouse gas, and its low boiling point influences its behavior in the atmosphere and during storage.
Factors Affecting Methane’s Boiling Point

- Pressure: Higher pressure increases methane’s boiling point, while lower pressure decreases it.
- Impurities: The presence of other gases or contaminants can alter methane’s boiling point.
- Temperature: Methane remains a gas above -161.5°C and liquefies below this temperature.
| Condition | Effect on Boiling Point |
|---|---|
| Increased Pressure | Higher Boiling Point |
| Decreased Pressure | Lower Boiling Point |
Practical Tips for Handling Methane

- Storage: Use insulated, cryogenic tanks to maintain methane in its liquid state.
- Transportation: Ensure tanks are pressure-regulated to prevent gasification.
- Safety: Handle methane in well-ventilated areas to avoid accumulation and potential hazards.
Methane’s Boiling Point in Commercial Applications

For businesses, understanding methane’s boiling point is essential for optimizing LNG production, reducing costs, and ensuring compliance with safety regulations. Companies investing in methane-related technologies can leverage this knowledge to enhance efficiency and sustainability.
Key Takeaways
- Methane’s boiling point is -161.5°C (-258.7°F).
- It is crucial for LNG production, storage, and environmental management.
- Factors like pressure and impurities affect its boiling point.
Checklist for Methane Handling
- [ ] Use cryogenic tanks for storage.
- [ ] Monitor pressure to maintain liquid state.
- [ ] Ensure safety protocols for handling methane.
What is methane's boiling point?
+Methane's boiling point is -161.5°C (-258.7°F) at standard atmospheric pressure.
Why is methane's boiling point important?
+It is crucial for LNG production, storage, and managing methane's environmental impact.
How does pressure affect methane's boiling point?
+Higher pressure increases the boiling point, while lower pressure decreases it.
In summary, methane’s boiling point is a fundamental property with wide-ranging implications for industry and the environment. Whether for informational or commercial purposes, mastering this concept ensures safer and more efficient use of methane. (methane properties, LNG production, greenhouse gases)



