Copper's Ground State Electron Configuration Explained Understanding Copper's Ground State Electron Setup Ground State Electron Configuration of Copper Simplified
Copper, a versatile and widely used metal, has a unique ground state electron configuration that sets it apart from other elements. Understanding this configuration is crucial for anyone studying chemistry, materials science, or even those interested in copper's applications in industries like electronics and construction. In this post, we’ll break down Copper's Ground State Electron Configuration in simple terms, explore its significance, and provide actionable insights for both informational and commercial audiences. Whether you're a student, researcher, or industry professional, this guide will help you grasp the essentials of copper’s electron setup, copper’s ground state electron configuration explained, and its practical implications.
What is Copper’s Ground State Electron Configuration?
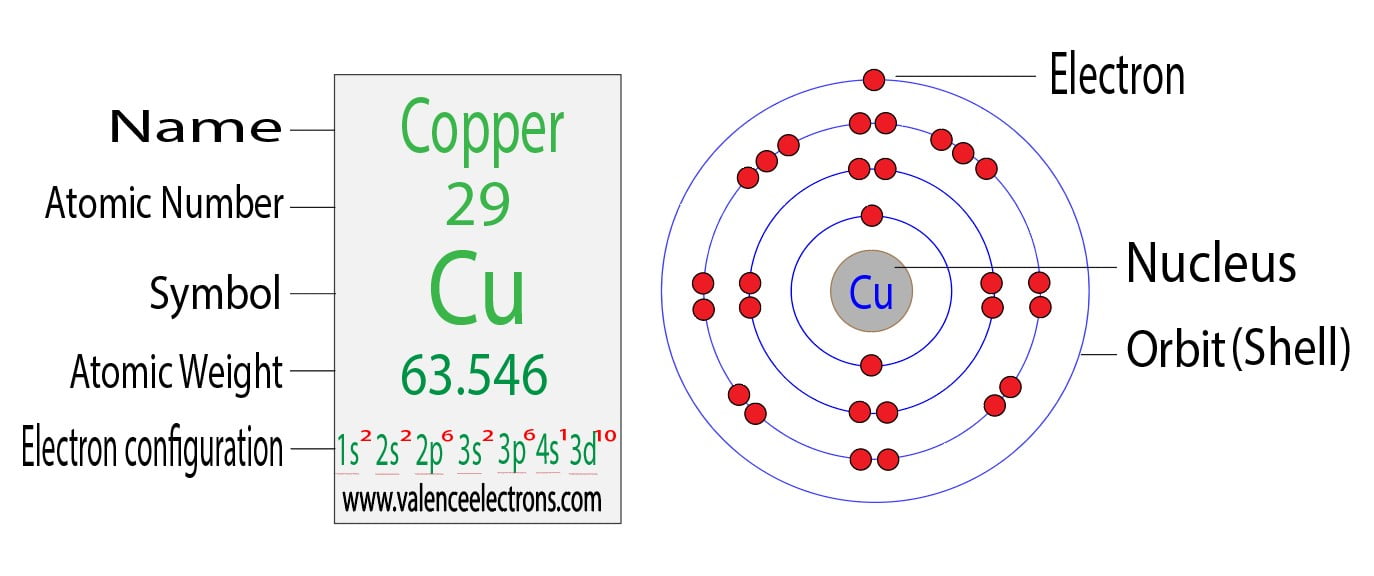
Copper (Cu) has an atomic number of 29, meaning it has 29 electrons in its neutral state. Its ground state electron configuration is [Ar] 3d^{10} 4s^1. This configuration is slightly unusual because it deviates from the expected order based on the Aufbau principle. Instead of having a [Ar] 3d^9 4s^2 configuration, copper adopts a more stable arrangement by promoting one 4s electron to the 3d orbital, resulting in a fully filled 3d subshell. This stability arises from the increased exchange energy in a full d-orbital, making it energetically favorable.
Why Copper’s Electron Configuration is Unique
Copper’s electron configuration is unique due to the half-filled and fully-filled subshell rule. The fully filled 3d^{10} subshell provides greater stability, which is why copper prefers this arrangement over the predicted 3d^9 4s^2. This uniqueness influences copper’s chemical properties, such as its high conductivity and resistance to corrosion, making it invaluable in various applications.
Practical Applications of Copper’s Electron Configuration
Understanding copper’s ground state electron setup is not just an academic exercise; it has real-world implications. Here’s how this knowledge applies to different fields:
1. Electronics and Conductivity
Copper’s 4s^1 electron is loosely bound, allowing it to move freely and conduct electricity efficiently. This property makes copper the preferred material for electrical wiring, circuits, and components. Its high conductivity ensures minimal energy loss, making it essential for sustainable energy solutions.
2. Industrial and Construction Uses
Copper’s stability and resistance to corrosion, influenced by its electron configuration, make it ideal for plumbing, roofing, and heat exchangers. Its durability ensures long-lasting performance in harsh environments, reducing maintenance costs and increasing efficiency.
| Application | Relevance to Electron Configuration |
|---|---|
| Electrical Wiring | Free movement of 4s^1 electron enables high conductivity. |
| Plumbing | Stability and corrosion resistance due to fully filled 3d^{10} subshell. |
| Renewable Energy | Efficient conduction for solar panels and wind turbines. |

📌 Note: Copper’s unique electron configuration is the foundation of its exceptional properties, making it a cornerstone material in modern technology.
In summary, copper’s ground state electron configuration, \[Ar\] 3d^{10} 4s^1, is key to its remarkable properties and wide-ranging applications. From electronics to construction, this understanding helps optimize its use in various industries. Whether you're exploring copper’s ground state electron configuration simplified or seeking commercial insights, this knowledge is invaluable. By grasping the basics of copper’s electron setup, you’re better equipped to appreciate its role in shaping our world, copper’s ground state electron configuration explained, understanding copper's ground state electron setup.
What is Copper’s Ground State Electron Configuration?
+
Copper’s ground state electron configuration is [Ar] 3d^{10} 4s^1, which deviates from the expected order to achieve greater stability.
Why is Copper’s Electron Configuration Unique?
+
It’s unique because it prioritizes a fully filled 3d^{10} subshell over the predicted 3d^9 4s^2, providing greater stability and influencing its properties.
How Does Copper’s Configuration Affect Its Conductivity?
+
The loosely bound 4s^1 electron allows for free movement, making copper an excellent conductor of electricity.